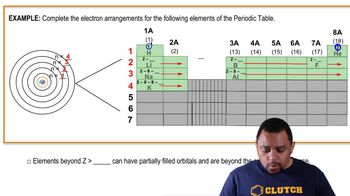
9. Electrons in Atoms and the Periodic Table
Electron Arrangements
9. Electrons in Atoms and the Periodic Table
Electron Arrangements
Practice this topic
- Multiple Choice
Write the electron arrangement for the following element:Calcium (Z = 20)
- Open QuestionHow many electrons are present in an atom in which the first and second shells and the 3s subshell are filled? Name the element.
- Open QuestionAn element has completely filled n = 1 and n = 2 shells and has six electrons in the n = 3 shell. Identify the element and its major group (i.e., main group, transition, etc.). Is it a metal or a nonmetal? Identify the orbital in which the last electron is found.
- Open QuestionFor chlorine, identify the group number, give the number of electrons in each occupied shell, and write its valence-shell configuration.