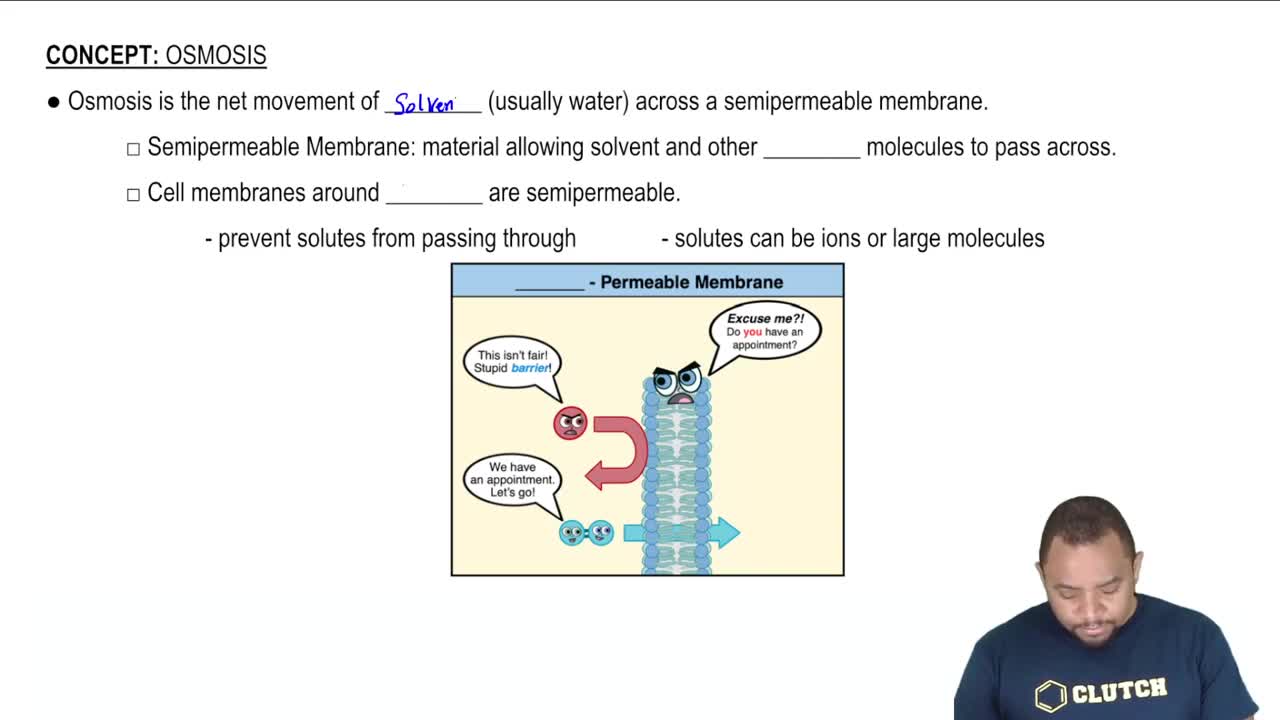
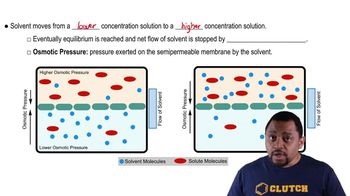
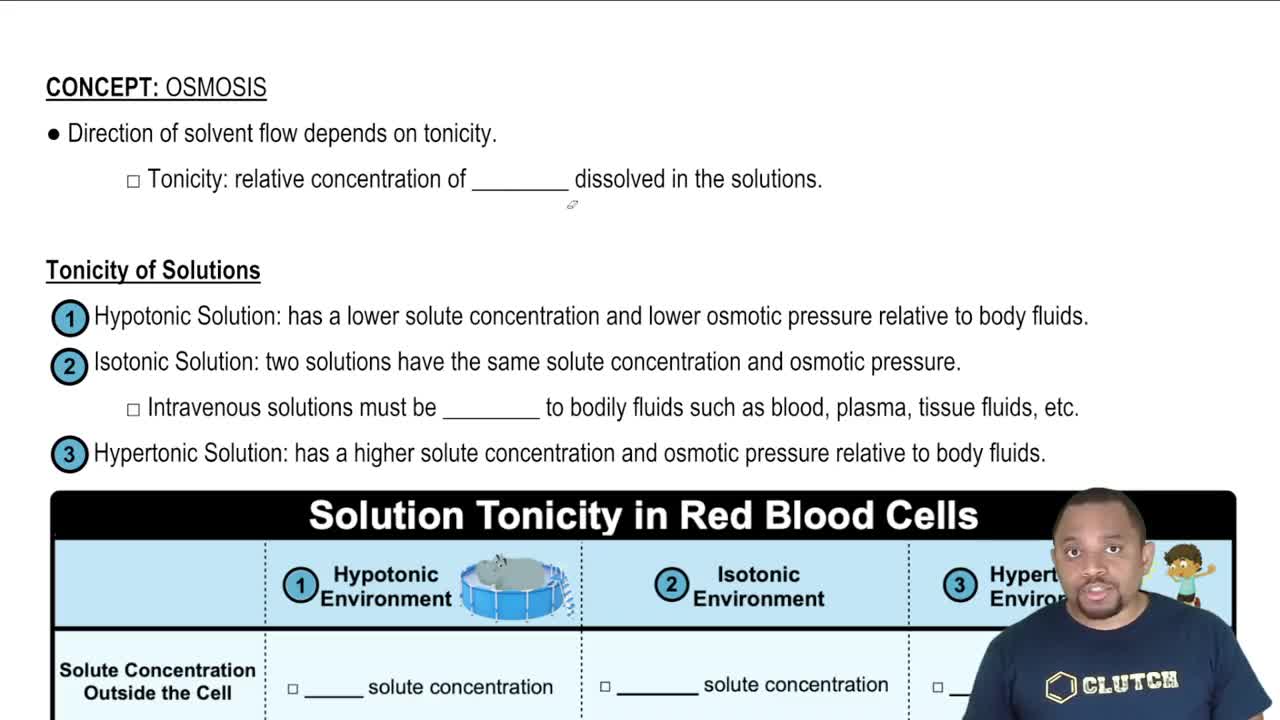
13. Solutions
Osmosis
13. Solutions
Osmosis
Practice this topic
- Multiple Choice
A semipermeable membrane is placed between the following solutions.
Which solution will increase in volume?
- Multiple Choice
Four U tubes each have distilled water in the right arm, a solution in the left arm, and a semipermeable membrane between the arms. If the solute is LiF, which solution is most concentrated?
- Multiple Choice
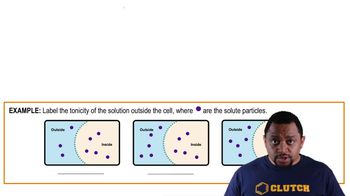
Identify the direction of water flow between 2 solutions separates by semipermeable membrane, where are the solute particles.
- Multiple Choice
If the fluid surrounding a patient's red blood cells is depleted in electrolytes, is crenation or hemolysis more likely to occur?
- Open QuestionAssume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a solution of a solute on the left side. Make a drawing that shows the situation after equilibrium is reached.
- Open QuestionWhat does it mean when we say that a 0.15 M NaCl solution is isotonic with blood, whereas distilled water is hypotonic?
- Open QuestionLook up the composition of Ringer's solution used in the treatment of burns and wounds.What is the osmolarity of the solution? Is it hypertonic, isotonic, or hypotonic with blood plasma (0.30 osmol)? Discuss possible medicinal reasons for the osmolarity of the solution.
- Open QuestionResearch information related to dialysis and answer the following questions:What is the difference between hemodialysis and peritoneal dialysis?