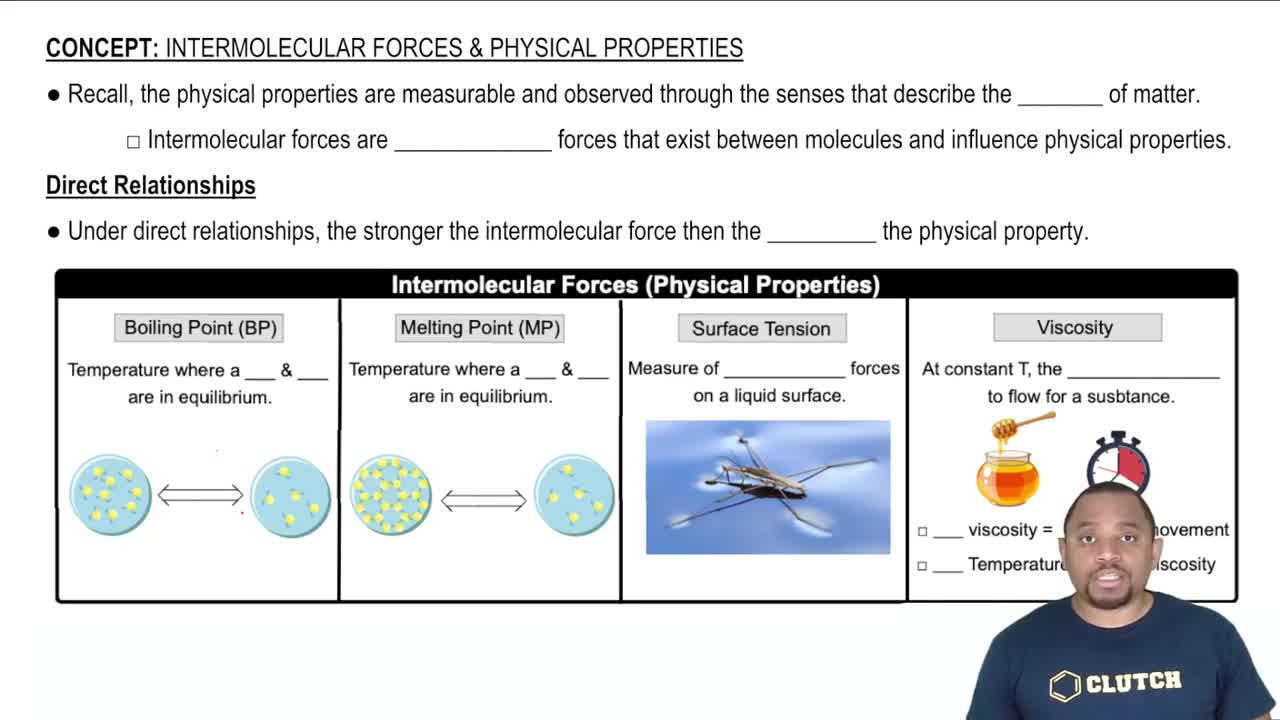
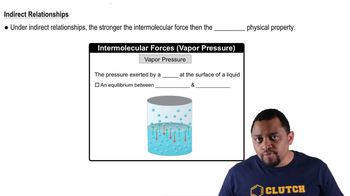
12. Liquids, Solids, and Intermolecular Forces
Intermolecular Forces and Physical Properties
12. Liquids, Solids, and Intermolecular Forces
Intermolecular Forces and Physical Properties
Practice this topic
- Multiple Choice
Which of the following will have the lowest boiling point?
- Multiple Choice
Which molecules would most likely cause a liquid to have the lowest viscosity?
- Multiple Choice
Which of the following should have the highest surface tension at a given temperature?
- Open QuestionWould you expect the boiling points to increase or decrease in the following series? Explain.Kr, Ar, Ne
- Open QuestionCompare the ∆Hvap values for water, isopropyl alcohol, ether, and ammonia, and order them from lowest to highest. Explain the rank order based on intermolecular attractive forces.
- Open QuestionWhat is the effect of pressure on a liquid's boiling point?