15. Chemical Equilibrium
Rate of Reaction
15. Chemical Equilibrium
Rate of Reaction
Showing 7 of 7 videos
Practice this topic
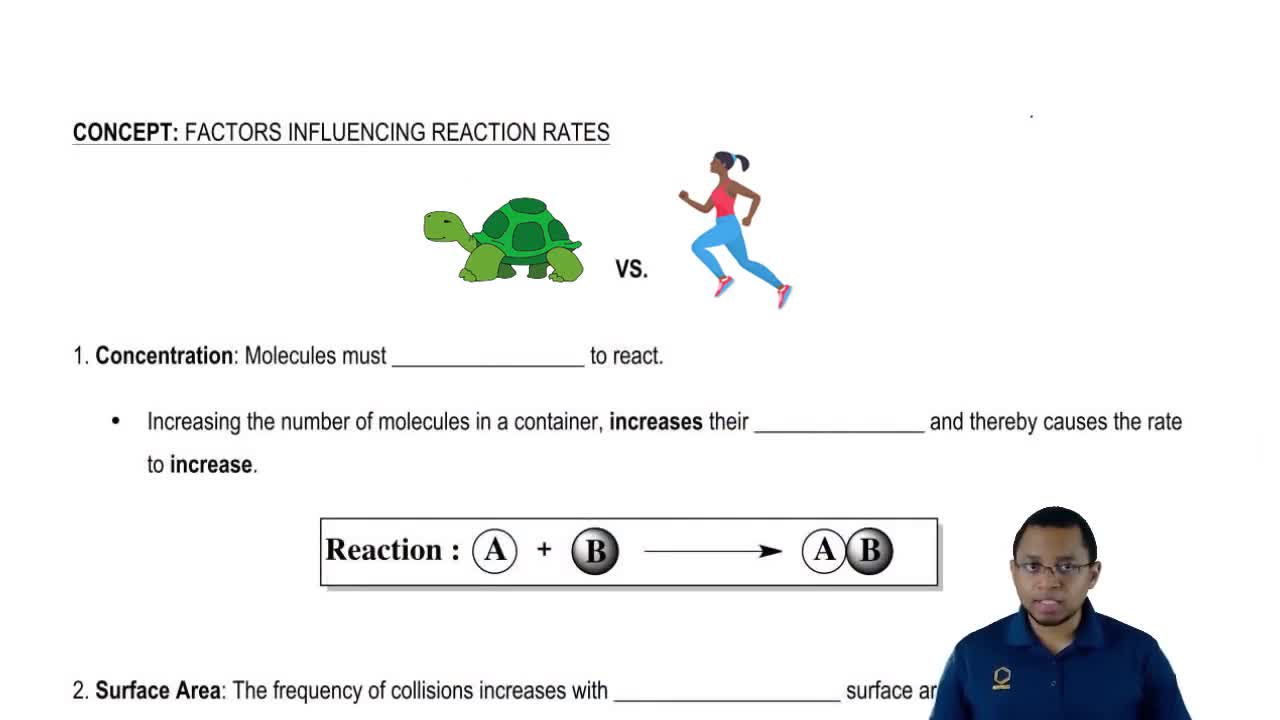
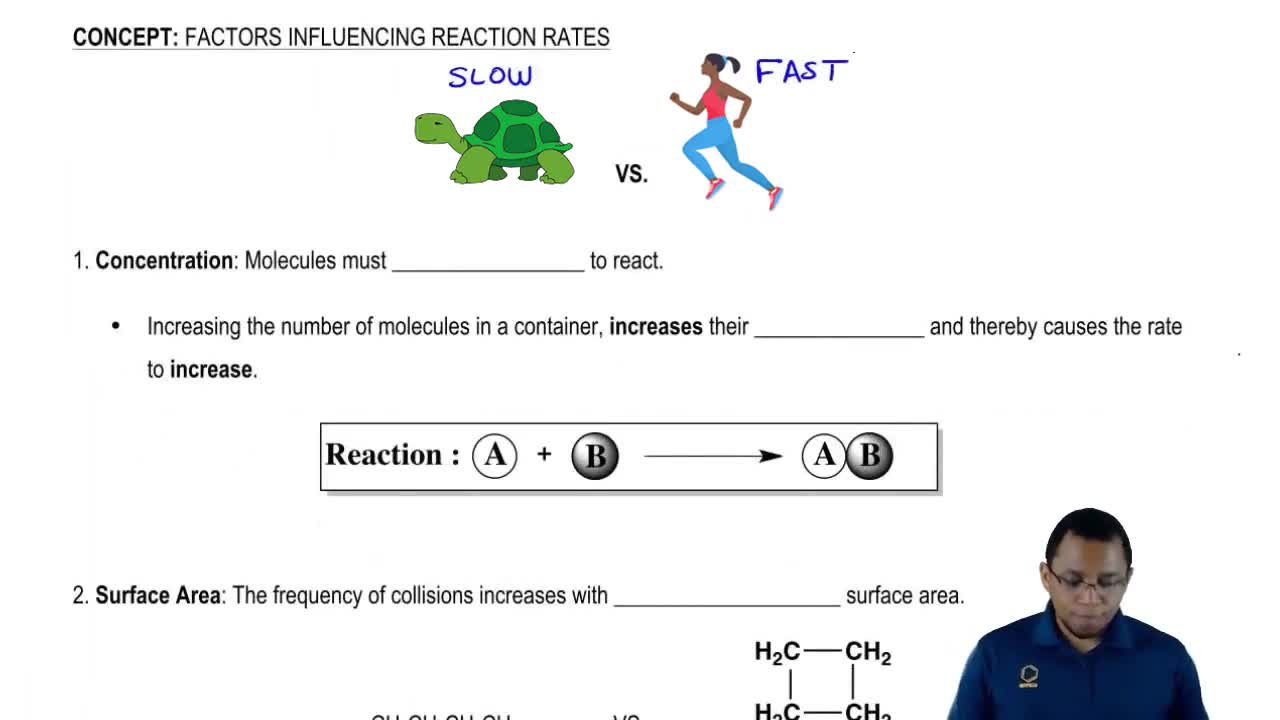
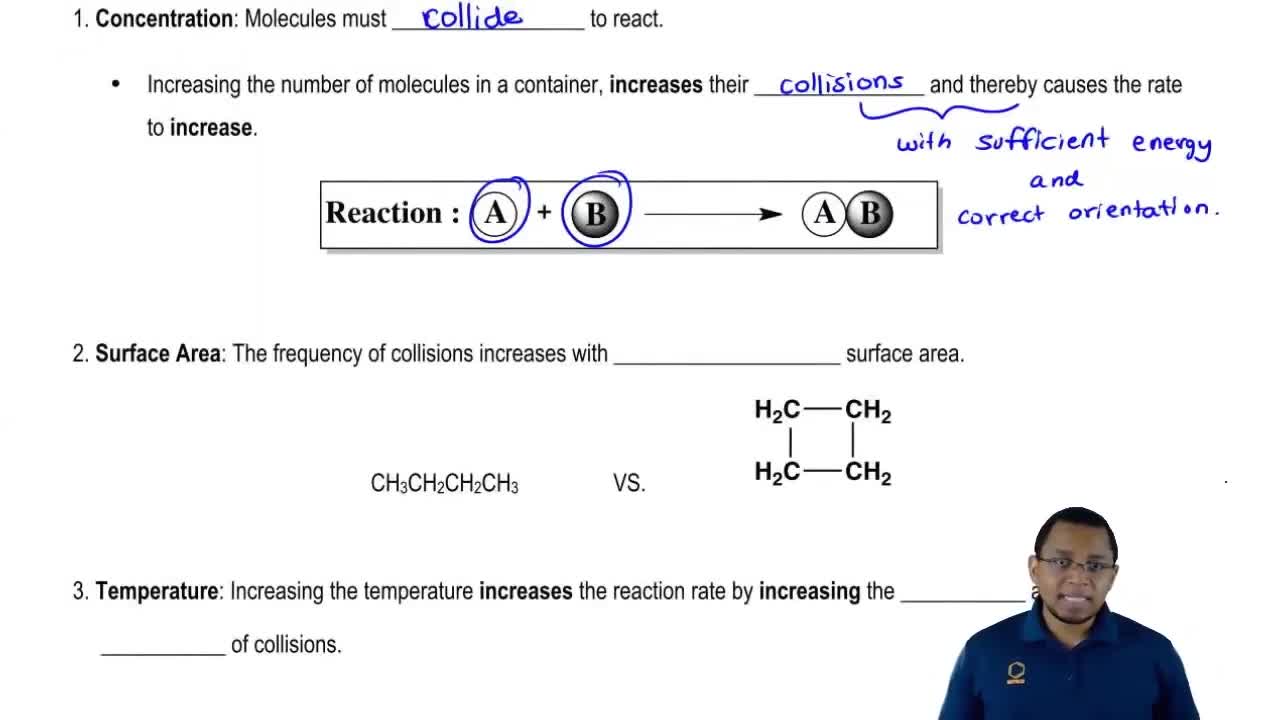
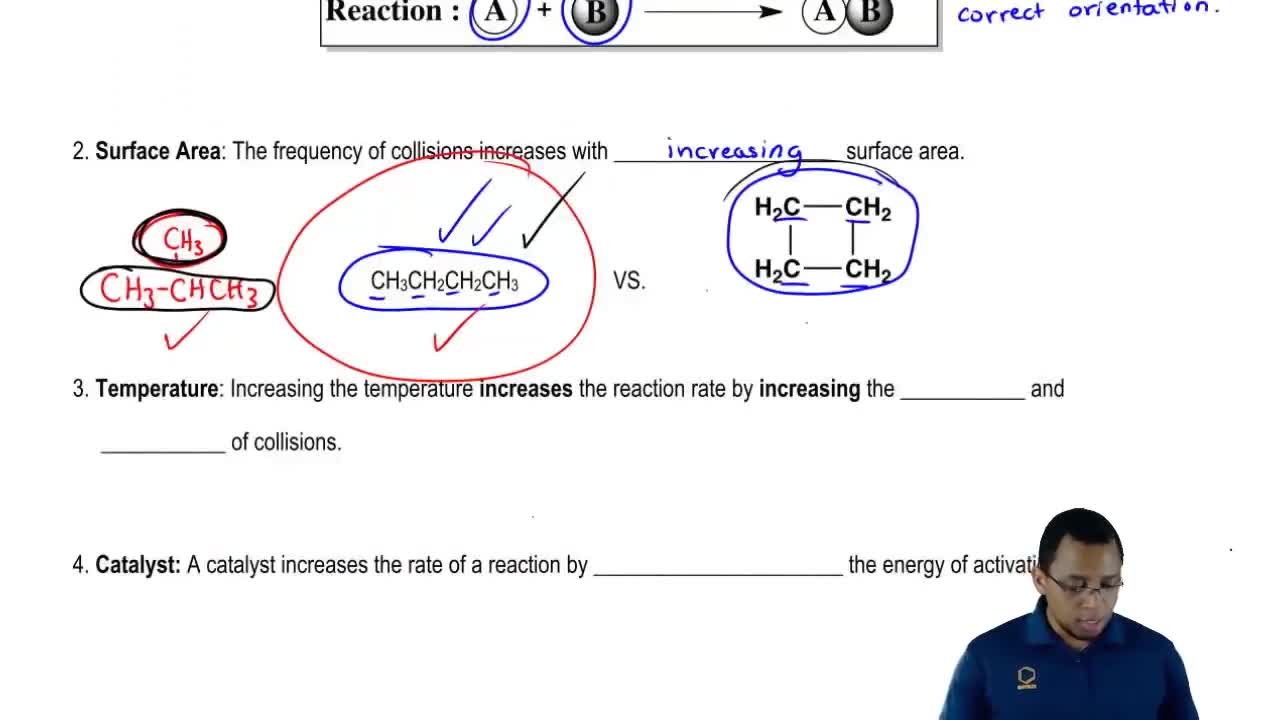
- Open QuestionWhy does increasing concentration generally increase the rate of a reaction?
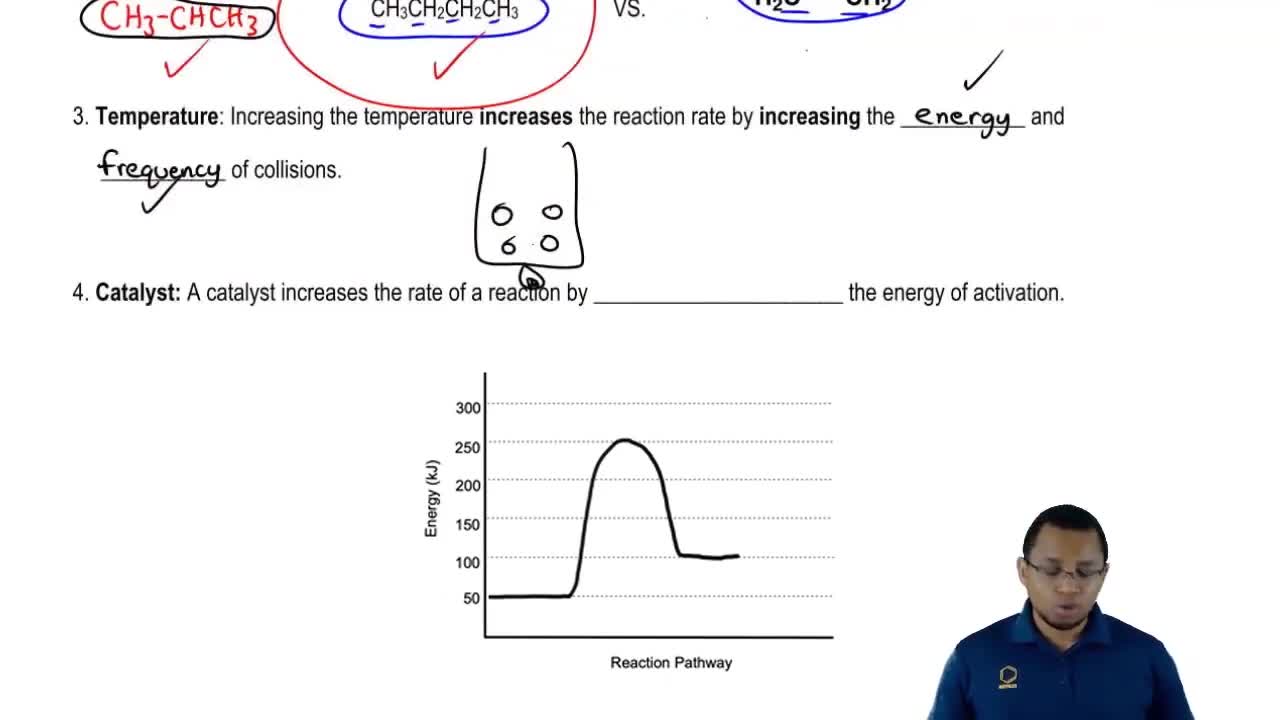
- Open QuestionFor the reaction C(s, diamond) → C(s, graphite), ∆G = -0.693 kcal/mol (-2.90 kJ/mol) at 25 °C.If a catalyst changes the activation energy of a forward reaction from 28.0 kcal/mol to 23.0 kcal/mol, what effect does it have on the reverse reaction?
- Open Questiona. What is meant by the rate of a reaction?
- Open QuestionHow would each of the following change the rate of the reaction shown here?2SO₂(g) + O₂(g) → 2SO₃(g)a. adding some SO₂(g)