7. Chemical Reactions
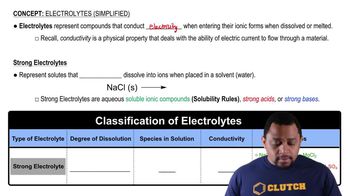
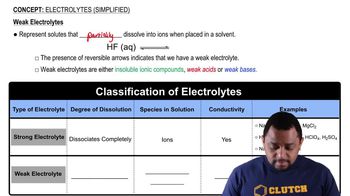
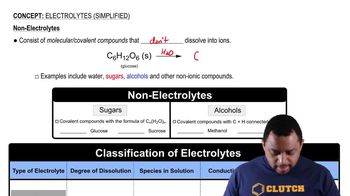
Electrolytes (Simplified)
7. Chemical Reactions
Electrolytes (Simplified)
Showing 6 of 6 videos
Practice this topic
- Open Question
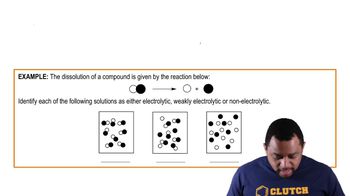
Each of the following reactions depicts a solute dissolving in water. Classify each solute as a strong electrolyte, a weak electrolyte or a non-electrolyte.
- Multiple Choice
Which of the following represents a non-electrolyte?
a) (CH3)2NH2 b) NaOH c) HIO3 d) C2H5OH e) CsNH2
- Open QuestionKF is a strong electrolyte, and HF is a weak electrolyte. How is the solution of KF different from that of HF?
- Open QuestionWrite a balanced equation for the dissociation of each of the following strong electrolytes in water:d. Fe(NO₃)₃
- Open QuestionIndicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:a. acetic acid, HC₂H₃O₂, a weak electrolyte