16. Oxidation and Reduction
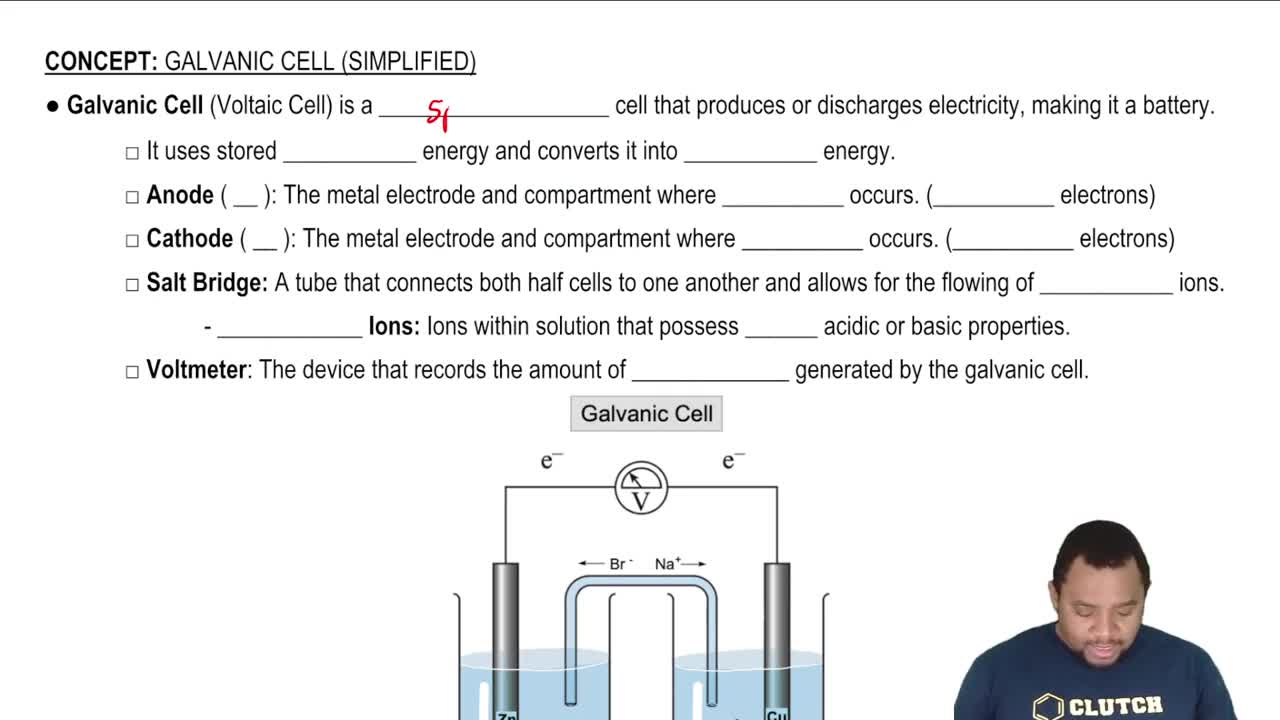
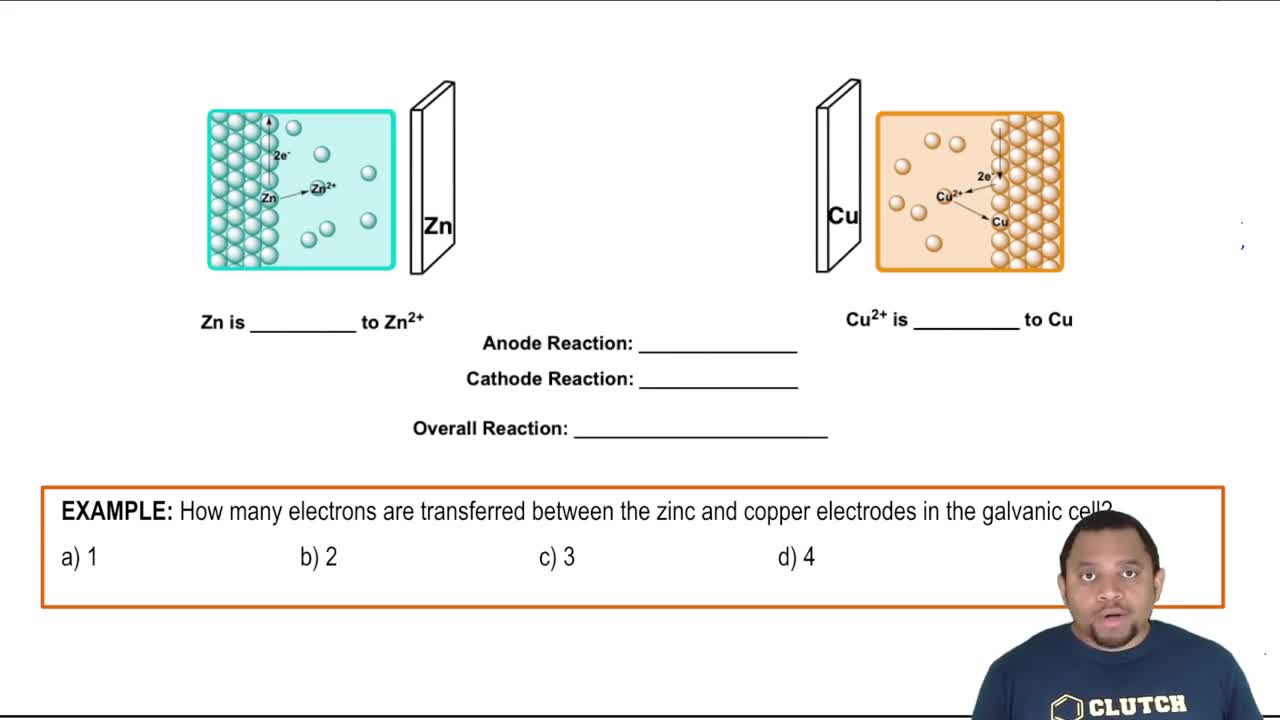
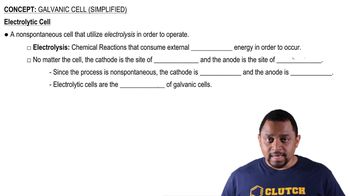
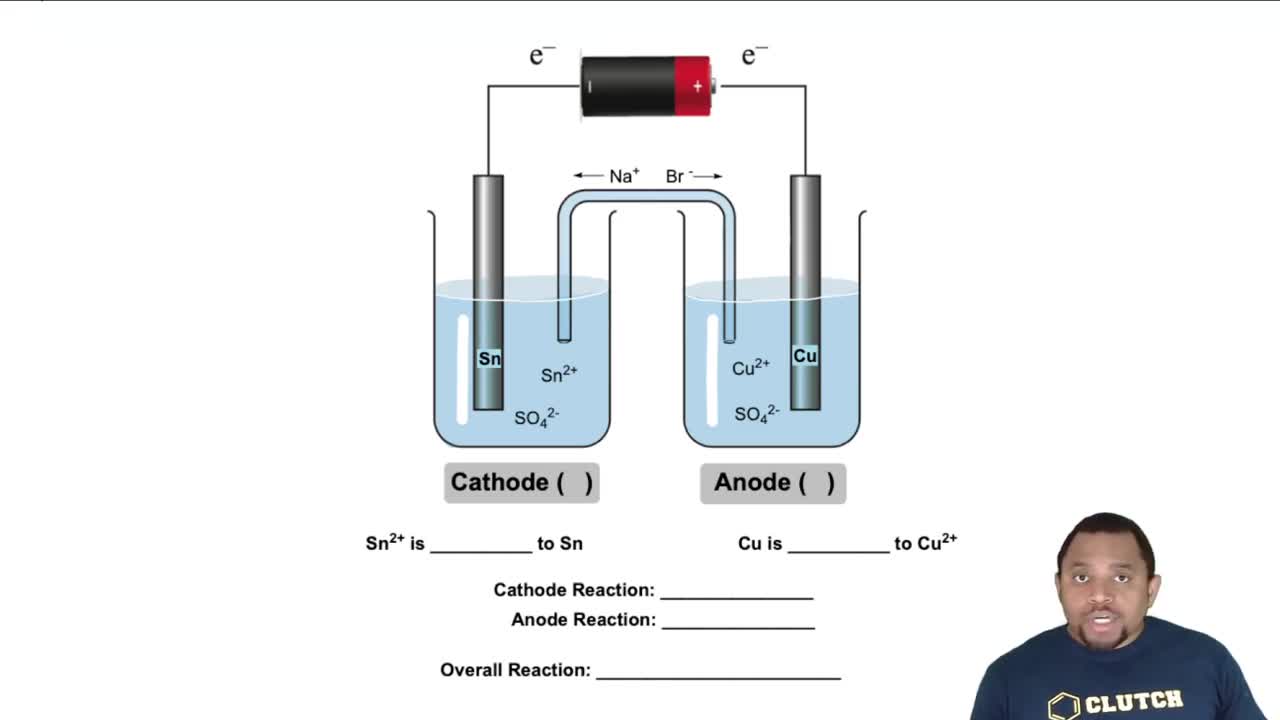
Galvanic Cell (Simplified)
16. Oxidation and Reduction
Galvanic Cell (Simplified)
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which of the following statements is true for a salt bridge?
- Multiple Choice
Which of the following statements is TRUE for a voltaic cell, but FALSE for an electrolytic cell?
I. The flow of electrons is spontaneous.
II. Oxidation occurs at the anode.
III. Electrons flow from the anode to the cathode. - Multiple Choice
What is the balanced half reaction that occurs at the anode in the overall cell reaction of a voltaic cell?
3 MnO4–(aq) + 5 Fe(s) → 3 Mn2+(aq) + 5 Fe3+(aq)