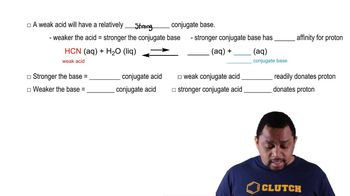
14. Acids and Bases
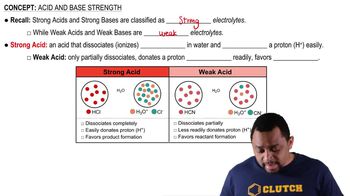
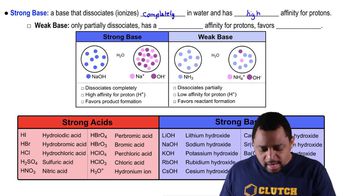
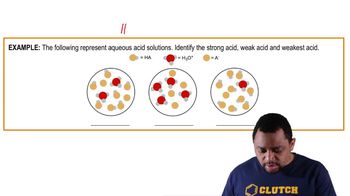
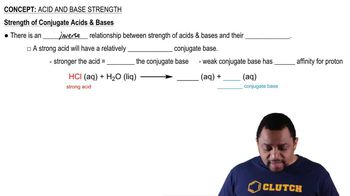
Acid and Base Strength
14. Acids and Bases
Acid and Base Strength
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Which of the following is the strongest base?
- Multiple Choice
Which of the following bases will have the weakest conjugate acid?
- Multiple Choice
Which of the following aqueous species will contain mostly reactants?
- Open Question
Determine [OH–] in each base solution. If the base is weak, indicate the value that [OH–] is less than.
- Multiple Choice
Predict the direction of the following reaction:
HC2H3O2 (aq) + H2O (liq) ______________ H3O+ (aq) + C2H3O2– (aq)
- Open QuestionWrite a balanced equation for the proton transfer reaction between hydrofluoric acid (HF) and ammonia (NH₃). Identify each conjugate acid-base pair, and rewrite the equilibrium arrows to indicate if the forward or reverse reaction is favored.
- Open QuestionFrom this electrostatic potential map of the amino acid alanine, identify the most acidic hydrogens in the molecule:
- Open QuestionElectrostatic potential maps of acetic acid (CH₃CO₂H) and ethyl alcohol (CH₃CH₂OH) are shown. Identify the most acidic hydrogen in each, and tell which of the two is likely to be the stronger acid.