9. Electrons in Atoms and the Periodic Table
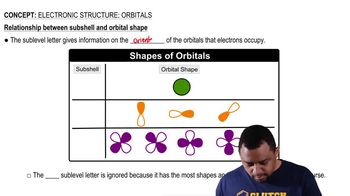
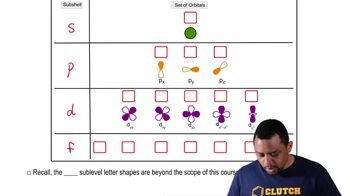
Electronic Structure: Orbitals
9. Electrons in Atoms and the Periodic Table
Electronic Structure: Orbitals
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Which of the following orbitals possesses the most orbital shapes?
- Multiple Choice
Which of the following statements is false?
a) A set of d orbitals contains 5 orbitals.
b) A set of 4s orbitals would have more energy than a set of 3p orbitals.
c) The second shell of an atom possesses d orbitals.
d) A set of f orbitals contains 3 orbitals.
e) The first energy level contains only s orbitals.