3. Matter and Energy
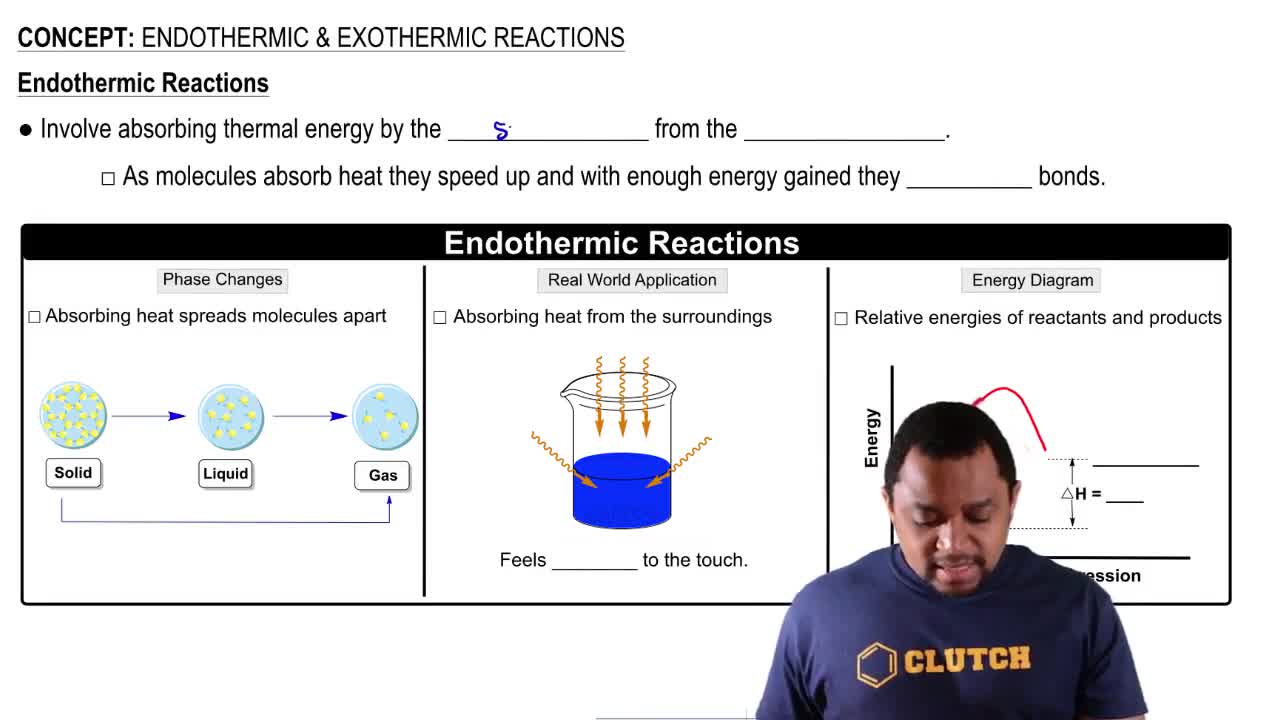
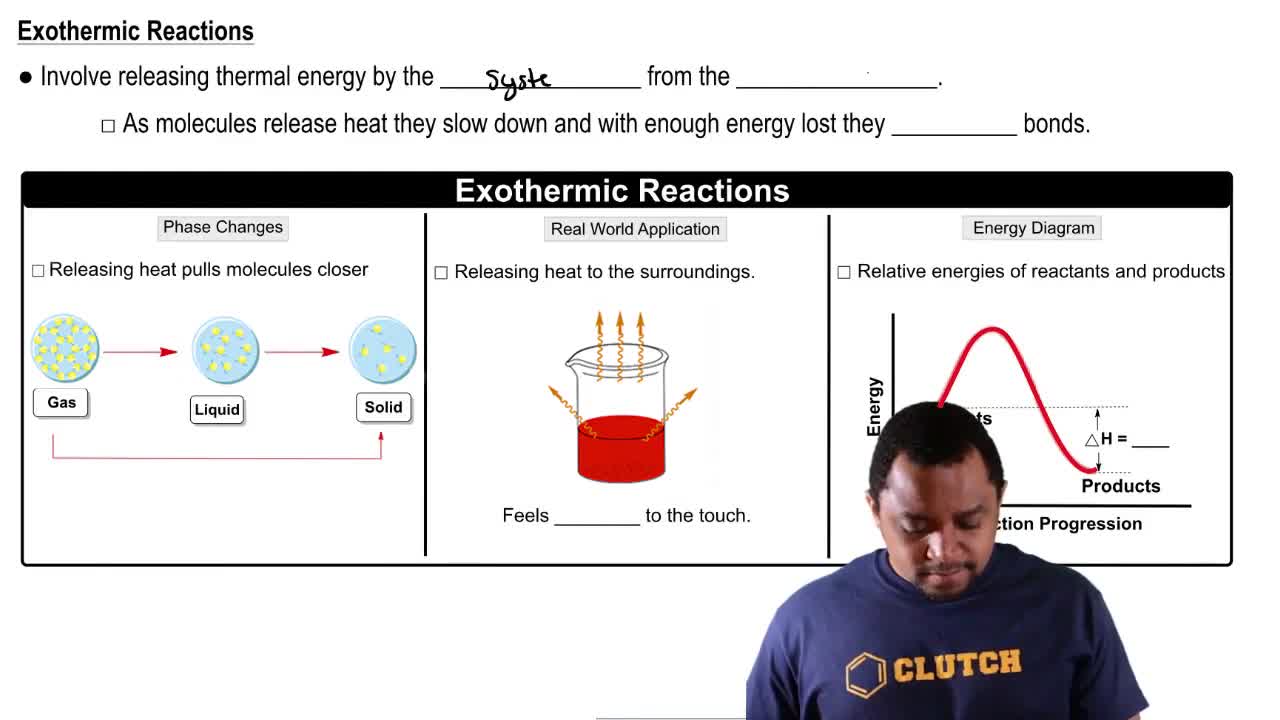
Endothermic & Exothermic Reactions
3. Matter and Energy
Endothermic & Exothermic Reactions
Practice this topic
- Open QuestionIn photosynthesis, green plants convert carbon dioxide and water into glucose (C6H12O6) according to the following equation:6 CO2(g) + 6 H2O(l) → C6H12O6(aq) + 6 O2(g)Is the reaction endothermic or exothermic?
- Open QuestionIs the total enthalpy (H) of the reactants for an endothermic reaction greater than or less than the total enthalpy of the products?
- Open QuestionThe vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol).What is the sign of ∆H for this process? Write a reaction showing heat as a product or reactant.
- Open QuestionOxygen can be converted into ozone by the action of lightning or electric sparks: 3 O2(g) ⇌ 2 O3(g)For this reaction, ∆H = +69kcal/mol (+285 kj/mol) and K = 2.68 x 10^-29 at 25 °C.Is the reaction exothermic or endothermic?