4. Atoms and Elements
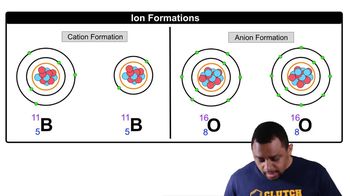
Ions (Simplified)
4. Atoms and Elements
Ions (Simplified)
Practice this topic
- Multiple Choice
Give the correct number of protons, neutrons and electrons for the following isotope:.
- Multiple Choice
In which pair are the two species both isoelectronic and isotopic?
- Multiple Choice
One isotope of a metallic element has a mass number of 65 and 35 neutrons in the nucleus. The cation that this atom forms has 28 electrons. What is the symbol of the cation?
- Multiple Choice
Which of the following is the symbol for the ion with a +4 charge, 30 neutrons and 21 electrons?
- Open Question
Fill in the gaps for the following table.
- Open QuestionWrite the symbol for the ion of each of the following:b. barium
- Open QuestionHow do atoms of different elements differ?
- Open QuestionWrite equations for the loss of an electron by a K atom and the gain of an electron by a K+ ion.