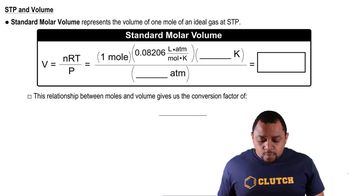
11 Gases
Standard Temperature and Pressure
11 Gases
Standard Temperature and Pressure
Practice this topic
- Multiple Choice
A sample of dichloromethane gas (CH2Cl2) occupies 32.6 L at 310 K and 5.30 atm. Determine its volume at STP?
- Multiple Choice
Which gas sample has the greatest volume at STP?
- Multiple Choice
Nitrogen and hydrogen combine to form ammonia via the following reaction:
1 N2 (s) + 3 H2 (g) → 2 NH3 (g)
What mass of nitrogen is required to completely react with 800.0 mL H2 at STP? - Open QuestionUsing the answer from problem 8.61, how many grams of nitrogen are in Whitney's lungs at STP if air contains 78% nitrogen?
- Open QuestionA gas has a volume of 2.84 L at 1.00 atm and 0 °C. At what temperature does it have a volume of 7.50 L at 520 mmHg?
- Open QuestionHow many molecules are in 1.0 L of O₂ at STP? How may grams of O₂?
- Open QuestionWhat is the mass of CH₄ in a sample that occupies a volume of 16.5 L at STP?