11 Gases
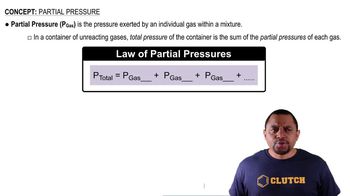
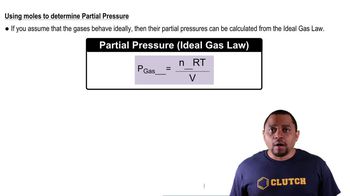
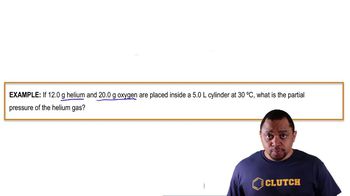
Dalton's Law: Partial Pressure (Simplified)
11 Gases
Dalton's Law: Partial Pressure (Simplified)
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
A gas mixture contains 72.8% chlorine and 27.2% neon by mass. What is the partial pressure of neon in the mixture if the total pressure is recorded as 809 mmHg?
- Multiple Choice
The partial pressure of N2 in the air is 593 mmHg at 1 atm. What is the partial pressure of N2 in a bubble of air a scuba diver breathes when he is 66 ft below the surface of the water where the pressure is 3.00 atm?
- Open QuestionDetermine the percent composition of air in the lungs from the following composition in partial pressures: PN2 = 573 mmHg, PO2 = 100 mmHg, PCO2 = 40 mmHg, and PH2O = 47 mmHg; all at 37 °C and 1 atm pressure.
- Open QuestionA mixture of nitrogen (N₂) and helium has a volume of 250 mL at 30 °C and a total pressure of 745 mmHg. (8.5, 8.6, 8.7)a. If the partial pressure of helium is 32 mmHg, what is the partial pressure of the nitrogen?
- Open QuestionWhat is meant by partial pressure?