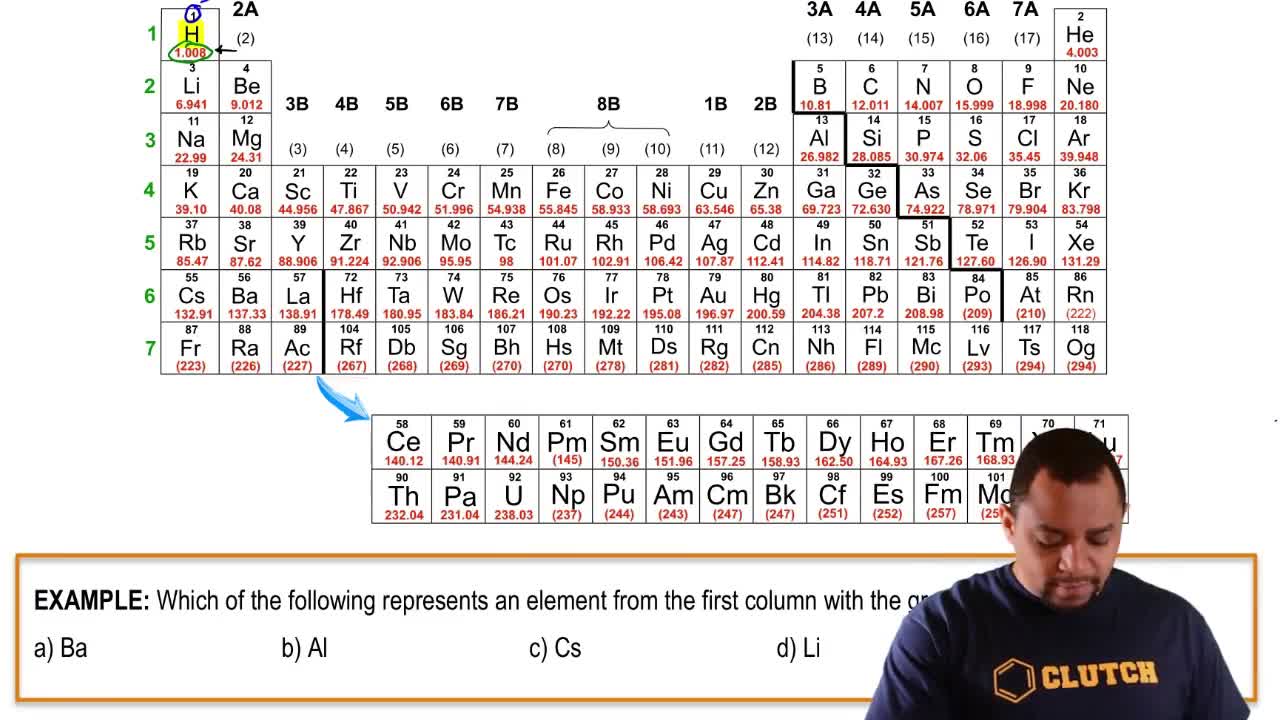
4. Atoms and Elements
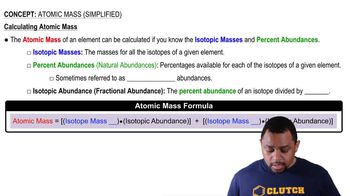
Atomic Mass (Simplified)
4. Atoms and Elements
Atomic Mass (Simplified)
Practice this topic
- Multiple Choice
Which of the following choices has the greatest atomic mass?
- Multiple Choice
Only three isotopes of magnesium exist on earth. 24Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25Mg has a 10.13% natural abundance, while 26Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. What is the mass of the 25Mg isotope?
- Multiple Choice
Silver has an atomic mass of 107.868 amu. The Ag-109 isotope (108.905 amu) is 48.16%. What is the amu of the other isotope?
- Open QuestionArgon has three naturally occurring isotopes, with mass numbers 36, 38, and 40.d. Why is the atomic mass of argon listed on the periodic table not a whole number?
- Open QuestionIndium consists of two isotopes, ¹¹³₄₉In and ¹¹⁵₄₉In. If the atomic mass for indium on the periodic table is 114.8, are there more atoms of ¹¹³₄₉In or ¹¹⁵₄₉In in a sample of indium?