12. Liquids, Solids, and Intermolecular Forces
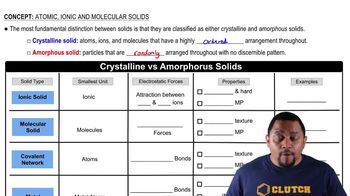
Atomic, Ionic and Molecular Solids
Practice this topic
- Multiple Choice
What is the major electrostatic force found within an ammonia molecule, NH3?
- Multiple Choice
As it cools off, olive oil slowly hardens and forms a solid over a range of temperatures. Which best describes it as a solid?
- Multiple Choice
Compound A is hard, doesn't conduct electricity, and melts at 1400ºC. Compound A represents which of the following:
- Multiple Choice
Classify each solid as amorphous, molecular, network covalent, alloy or ionic.
a) Steel ______________________
b) CO2 ______________________
c) Graphite ______________________
d) CaCO3 ______________________
e) Bronze, an alloy of Cu and Sn ______________________ - Open QuestionList three kinds of crystalline solids, and give an example of each.