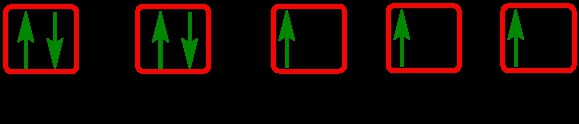
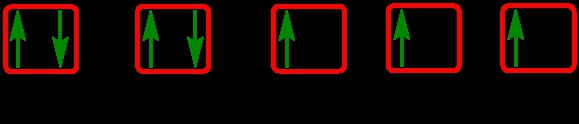
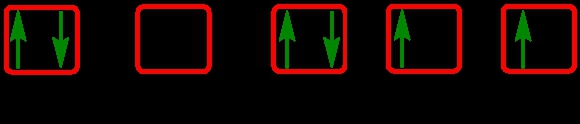
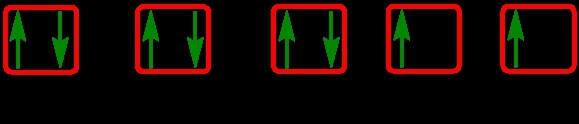
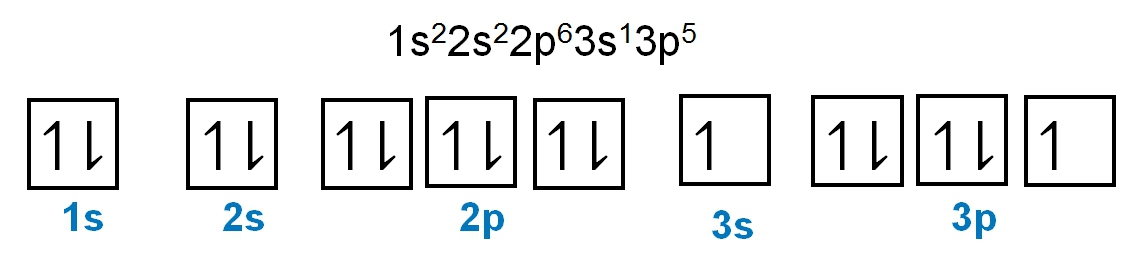
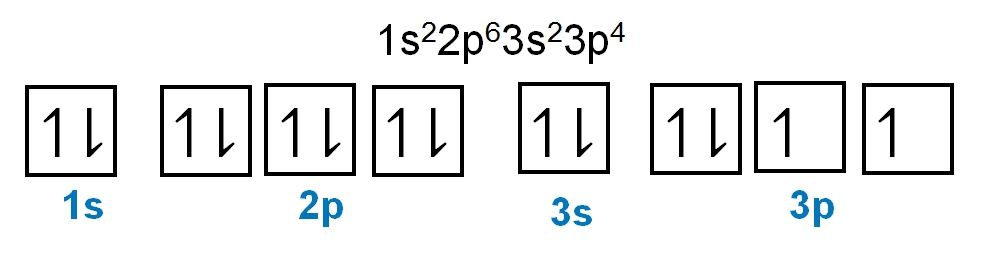
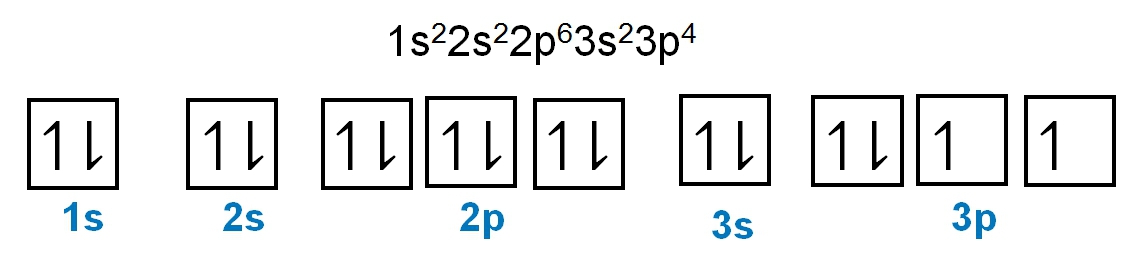
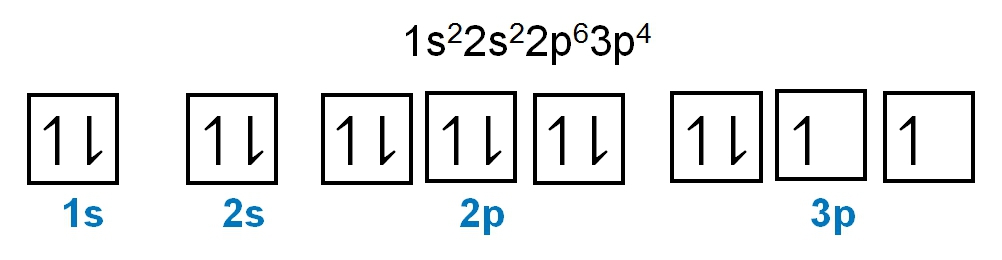
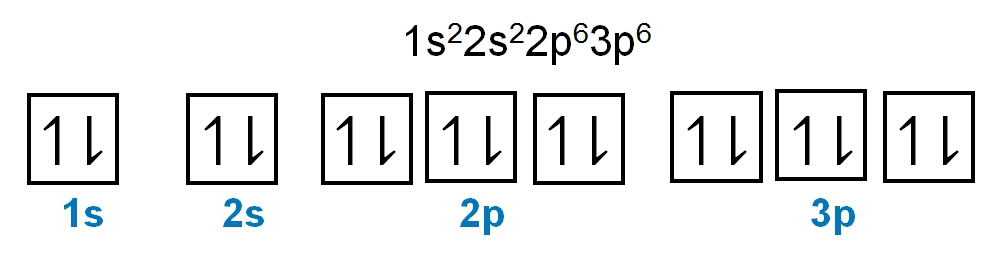
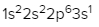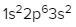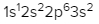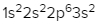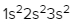The periodic law plays a crucial role in determining the electron arrangements of elements, which can be visually represented through electron orbital diagrams. These diagrams illustrate the distribution of electrons within various orbitals, highlighting the concept of degenerate orbitals—sets of orbitals that possess the same energy level. According to Hund's rule, these degenerate orbitals are filled in a specific manner: they are first half-filled before any orbital is completely filled.
To understand the filling of these orbitals, we can examine the different sublevels:
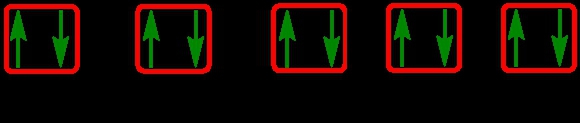
1. **s Sublevel**: The s sublevel contains 1 orbital and can hold a maximum of 2 electrons. In the orbital diagram, one electron spins up and the other spins down, representing the two possible states of the electrons.
2. **p Sublevel**: The p sublevel consists of 3 orbitals, allowing for a maximum of 6 electrons. Following Hund's rule, each of the three orbitals is first half-filled with one electron (all spinning up) before any orbital receives a second electron (spinning down).
3. **d Sublevel**: The d sublevel has 5 orbitals, accommodating a total of 10 electrons. Similar to the p sublevel, the d orbitals are half-filled first, with one electron in each orbital before pairing occurs.
4. **f Sublevel**: The f sublevel contains 7 orbitals and can hold up to 14 electrons. Again, according to Hund's rule, these orbitals are half-filled first before any are completely filled.
In summary, the maximum number of electrons that can be held in each sublevel is as follows: the s sublevel can hold 2 electrons, the p sublevel can hold 6, the d sublevel can hold 10, and the f sublevel can hold 14. Understanding these principles is essential for grasping the electron configuration of elements and their chemical behavior.