5. Molecules and Compounds
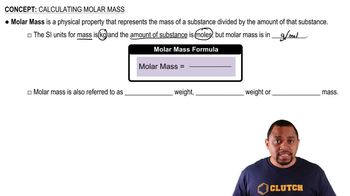
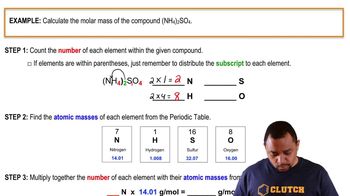
Calculating Molar Mass
5. Molecules and Compounds
Calculating Molar Mass
Practice this topic
- Multiple Choice
Calculate the molecular weight of C3H5N3O3.
- Multiple Choice
The reaction between nickel metal and hydrochloric acid is not a simple dissolution. The product formed is NiCl2 • 6 H2O (s), nickel (II) chloride hexahydrate, which has exactly 6 waters of hydration in the crystal lattice for every nickel ion. What is the molar mass of nickel (II) chloride hexahydrate, NiCl2 • 6 H2O (s)?
- Multiple Choice
What is the molar mass of diazepam also known as Valium if 0.05570 mol weighs 15.86 g?
- Open QuestionCalculate the molar mass for each of the following:b. C₃H₆O₃
- Open QuestionCalculate the molar mass for each of the following:c. Fe(ClO₄)₃
- Open QuestionCalculate the molar mass for each of the following:a. Al₂(SO₄)₃, antiperspirant
- Open QuestionLovastatin, a drug used to lower serum cholesterol.Look up the molecular formula for Lovastatin and calculate the molar mass.