13. Solutions
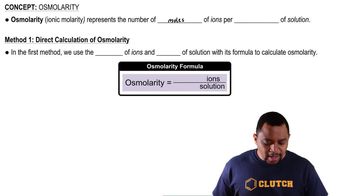
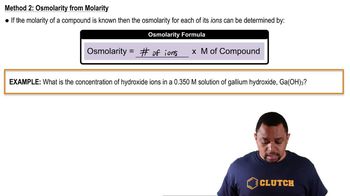
Osmolarity
13. Solutions
Osmolarity
Practice this topic
- Multiple Choice
Which of the following solutions will have the highest concentration of bromide ions?
- Multiple Choice
How many milligrams of nitride ions are required to prepare 820 mL of 0.330 M Ba3N2 solution?
- Multiple Choice
How many bromide ions are present in 65.5 mL of 0.210 M GaBr3 solution?
- Open QuestionWhat is the osmolarity of the following solutions?a. 0.35 M KBrb. 0.15 M glucose + 0.05 M K₂SO₄
- Open QuestionWhich of the following solutions has the higher osmolarity?0.30 M NaOH or 3.0% (m/v) NaOH
- Open Questionn isotonic solution must be approximately 0.30 osmol/L. How much KCl is needed to prepare 175 mL of an isotonic solution?