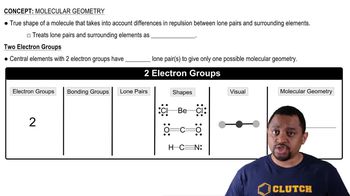
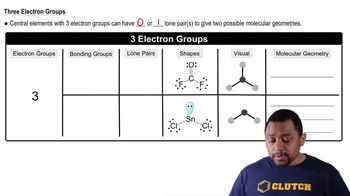
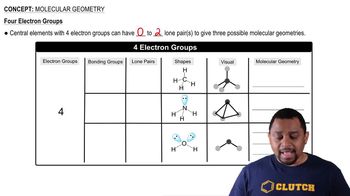
10. Chemical Bonding
Molecular Geometry (Simplified)
10. Chemical Bonding
Molecular Geometry (Simplified)
Showing 5 of 5 videos
Practice this topic
- Open QuestionChoose the shape (1 to 6) that matches each of the following descriptions (a to c): 1. linear2. bent (109°)3. trigonal planar4. bent (120°)5. trigonal pyramidal6. tetrahedralb. a molecule with a central atom that has four electron groups and three bonded atoms
- Open QuestionCompare the Lewis structures of CF₄ and NF₃ Why do these molecules have different shapes?
- Open QuestionThe phosphonium ion, PH⁺₄ is formed by reaction of phosphine, PH₃ , with an acid. b. Predict its molecular geometry.
- Open QuestionThe sulfite ion (SO₃²⁻) and sulfur trioxide (SO₃) have the same chemical formulas but different molecular geometries. Draw the Lewis dot structures and identify the molecular geometry of each.