2. Measurement and Problem Solving
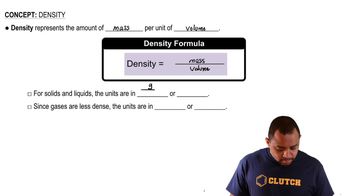
Density
2. Measurement and Problem Solving
Density
Practice this topic
- Multiple Choice
When lead levels in blood exceed 0.80 ppm (parts per million) the level is considered dangerous. 0.80 ppm means that 1 million g of blood would contain 0.80 g of Pb. Given that the density of blood is 1.060 kg/cm3, how many grams of Pb would be found in 400.00 mL of blood at 0.620 ppm?
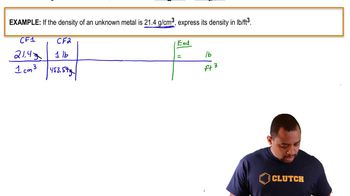
- Open QuestionDetermine the density (g/mL) for each of the following:b. A cube of butter weighs 0.250 lb and has a volume of 130.3mL.
- Open QuestionDetermine the density (g/mL) for each of the following:a. A 20.0-mL sample of a salt solution has a mass of 24.0 g.
- Open QuestionWhat is the density (g/mL) of each of the following samples?b. A syrup is added to an empty container with a mass of 115.25 g. When 0.100 pt of syrup is added, the total mass of the container and syrup is 182.48 g.
- Open QuestionWhat is the density (g/mL) of each of the following samples?b. A 14.3 - cm³ sample of tin has a mass of 0.104 kg.