17. Radioactivity and Nuclear Chemistry
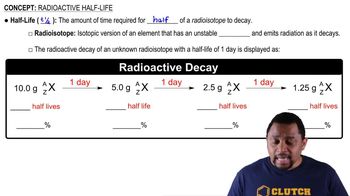
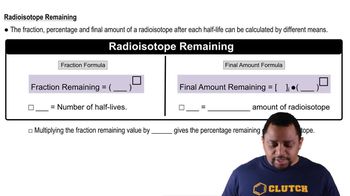
Radioactive Half-Life
17. Radioactivity and Nuclear Chemistry
Radioactive Half-Life
Practice this topic
- Multiple Choice
The half-life of arsenic-74 is about 18 days. If a sample initially contains 100.00 mg arsenic-74, what mass (in mg) would be left after 72 days?
- Multiple Choice
The half-life of iodine-131, an isotope used in thyroid therapy, is 8.021 days. What percentage of iodine-131 remains in a sample that is estimated to be 40.11 days old?
- Multiple Choice
What is the concentration of a CO2 in a container after 4 half-lives if 0.325 mol of CO2 is initially placed into a 5.0 L reaction vessel?
- Multiple Choice
What is the half-life of the radioisotope that shows the following plot of remaining percentage vs. time?
- Open QuestionWhat is wrong with the following decay curve? Explain.
- Open QuestionWhat does it mean when we say that strontium-90, a waste product of nuclear power plants, has a half-life of 28.8 years?
- Open QuestionHarmful chemical spills can often be cleaned up by treatment with another chemical. For example, a spill of H₂SO₄ might be neutralized by addition of NaHCO₃. Why is it that the harmful radioactive wastes from nuclear power plants cannot be cleaned up as easily?