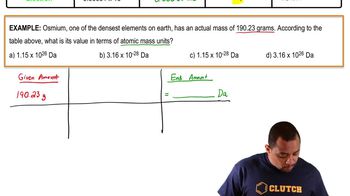
4. Atoms and Elements
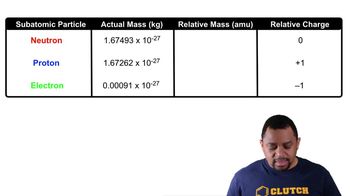
Subatomic Particles (Simplified)
4. Atoms and Elements
Subatomic Particles (Simplified)
Practice this topic
- Multiple Choice
According to the table above, how many electrons are needed to have a combined mass of 1.0465 x 10-25 kg?
- Multiple Choice
How many atoms are contained in 0.230 g of sodium, Na? The mass of one sodium atom is 23.99 amu.
- Open QuestionIs each of the following statements true or false?c. Neutrons repel each other.
- Open QuestionHow many protons and electrons are there in a neutral atom of each of the following elements?a. argon
- Open QuestionWhich statements completed with a to e will be true and which will be false? An atom of N compared to an atom of Li has a larger (greater)c. number of protons