2. Measurement and Problem Solving
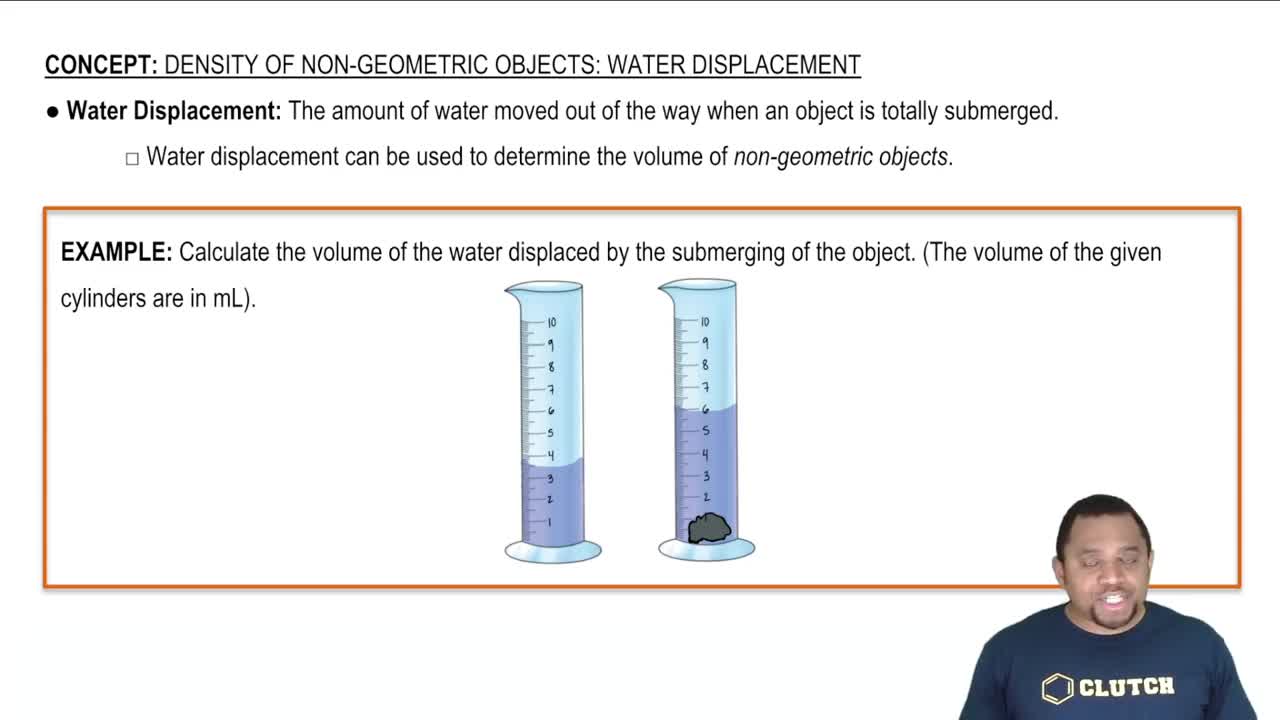
Density of Non-Geometric Objects
2. Measurement and Problem Solving
Density of Non-Geometric Objects
Practice this topic
- Multiple Choice
A piece of unknown solid weighs approximately 0.045 lbs. When a scientist places it in a glass beaker the water level increases from 200 mL to 260 mL. What is the density of the unknown solid in g/mL?
- Multiple Choice
If an irregularly shaped apple possesses a density of 0.96 g/cm3, what is its mass in milligrams? (The volume of the given cylinders are in mL).
- Open QuestionDetermine the density (g/mL) for each of the following:c. A gem has a mass of 4.50 g. When the gem is placed inagraduated cylinder containing 12.00 mL of water, thewater level rises to 13.45 mL.
- Open QuestionUse the density values in TABLE2.8 to solve each of the following problems:a. A graduated cylinder contains 18.0 mL of water. What is the new water level, in milliliters, after 35.6 g of silver metal is submerged in the water?
- Open QuestionThe water level in a graduated cylinder initially at 215 mL rises to 285 mL after a piece of lead is submerged. What is the mass, in grams, of the lead(see TABLE2.8)? (2.7)
- Open QuestionA graduated cylinder contains 155 mL of water. A 15.0-g piece of iron and a 20.0-g piece of lead are added. What is the new water level, in milliliters, in the cylinder(see TABLE2.8)? (2.7)