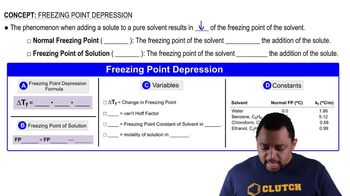
13. Solutions
Freezing Point Depression
13. Solutions
Freezing Point Depression
Practice this topic
- Multiple Choice
How many moles of ethylene glycol, C2H6O2, must be added to 1,000 g of water to form a solution that has a freezing point of – 10ºC?
- Multiple Choice
An ethylene glycol solution contains 28.3 g of ethylene glycol, C2H6O2 in 97.2 mL of water. Calculate the freezing point of the solution. The density of water 1.00 g/mL.
- Multiple Choice
When 825 g of an unknown is dissolved in 3.45 L of water, the freezing point of the solution is decreased by 2.89ºC. Assuming that the unknown compound is a non-electrolyte, calculate its molar mass.
- Open QuestionMany compounds are only partially dissociated into ions in aqueous solution. Trichloroacetic acid (CCl₃CO₂H), for instance, is partially dissociated in water according to the equationCCl₃CO₂H (aq) → H⁺ (aq) + CCl₃CO₂⁻ (aq)For a solution prepared by dissolving 1.00 mol of trichloroacetic acid in 1.00 kg of water, 36.0% of the trichloroacetic acid dissociates to form H⁺ and CCl₃CO₂⁻ ions.What is the freezing point of this solution? (The freezing point of 1 kg of water is lowered 1.86 °C for each mole of solute particles.)