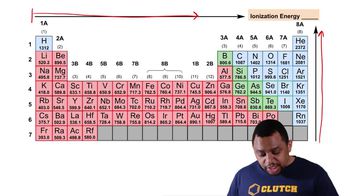
9. Electrons in Atoms and the Periodic Table
Periodic Trend: Ionization Energy (Simplified)
9. Electrons in Atoms and the Periodic Table
Periodic Trend: Ionization Energy (Simplified)
Practice this topic
- Multiple Choice
Rank the following elements in order of increasing ionization energy:Br, F, Ga, K and Se.
- Multiple Choice
Which of the following elements would lose an electron the easiest?
- Multiple Choice
Which element from Group 7A has lowest ionization energy.
- Multiple Choice
Which of the following has the highest ionization energy?
- Open QuestionWhich of the following ions are likely to form? Explain.a. Li^2+b. K-c. Mn^3+d. Zn^4+e. Ne+
- Open QuestionFill in the following blanks using larger or smaller, higher orlower. Mg has a atomic size and a ionization energy than Cs.
- Open QuestionWhich statements completed with a to e will be true and which will be false? An atom of N compared to an atom of Li has a larger (greater)b. ionization energy
- Open QuestionWhy is the ionization energy of Cl lower than F, but higher than that of S? (4.7)