Open Question
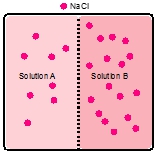
Assume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a solution of a solute on the left side. Make a drawing that shows the situation after equilibrium is reached.
 Verified step by step guidance
Verified step by step guidance