Multiple Choice
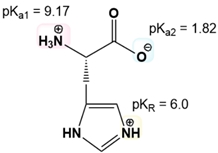
Draw Glu and calculate its isoelectric point. pKa1 = 9.67. pKa2 = 2.19. pKR = 4.25.
 Verified step by step guidance
Verified step by step guidance
 12:3m
12:3mMaster Isoelectric Point Of Amino Acids With Ionizable R-Groups with a bite sized video explanation from Jason
Start learning
