Enzyme kinetics is a vital area of biochemistry that focuses on the rates, or velocities, of enzyme-catalyzed reactions, denoted by the symbol v. Understanding the factors that influence these reaction rates is crucial for grasping how enzymes function within biological systems. One primary method to enhance reaction rates is by increasing the temperature. While this can initially boost reaction rates, it poses a risk to cells, as elevated temperatures can lead to the denaturation of proteins, ultimately causing cellular stress and death. A graph illustrating this relationship shows that reaction rates increase with temperature until a critical point is reached, beyond which proteins begin to lose their functional structure.
Another approach to increase reaction rates is by raising substrate concentration. However, this method is energy-intensive and time-consuming, as producing sufficient substrate can lead to overcrowding in the cellular environment. A graph depicting this scenario indicates that while reaction rates rise with substrate concentration, achieving significantly high rates requires an impractically large amount of substrate, which can be detrimental to cellular function.
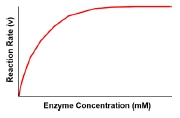
The most effective strategy for cells to enhance reaction rates is through the addition of catalysts, specifically enzymes. Enzymes are remarkable because they can dramatically increase reaction rates even in small quantities. A comparative graph shows that with the introduction of an enzyme, the reaction rate rises significantly at lower substrate concentrations, demonstrating the efficiency of enzymes in facilitating biochemical reactions. This method is preferred in living systems, as it allows for precise control over metabolic processes without the drawbacks associated with temperature or substrate concentration increases.
In summary, while temperature and substrate concentration can influence enzyme activity, the use of enzymes as catalysts stands out as the most efficient and effective means for cells to regulate and enhance reaction rates.