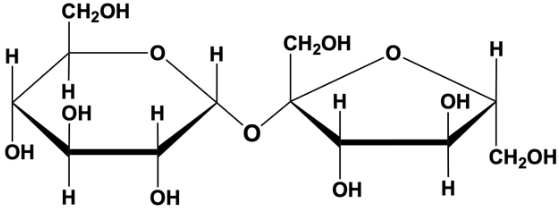
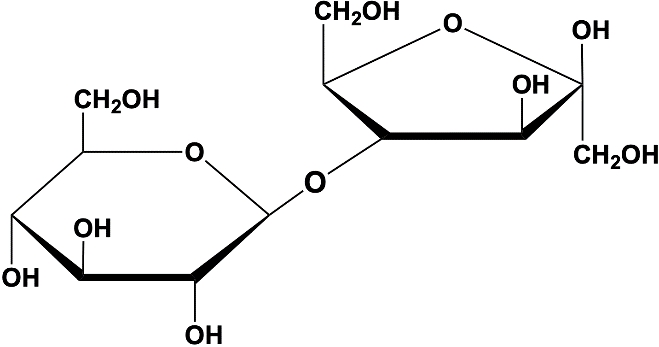
A glycosidic bond is a crucial linkage in biochemistry, defined as an acetal or ketal connection between a sugar's anomeric carbon and another chemical group. This other group can be another monosaccharide, enabling the formation of polysaccharides, or it can be a different type of molecule, such as a protein or lipid. Understanding glycosidic bonds is essential as they play a significant role in the structure and function of carbohydrates.
These bonds are formed through a process known as dehydration synthesis, which involves the removal of a water molecule. This reaction is illustrated by the interaction between two glucose molecules, one in the alpha configuration and the other in the beta configuration. When a water molecule is eliminated during this reaction, a glycosidic bond is created between the anomeric carbon of one sugar and the hydroxyl group of another, resulting in a compound referred to as a glycoside.
Conversely, glycosidic bonds can be broken through hydrolysis, which is the reverse of dehydration synthesis. In this reaction, water is added back to the molecule, cleaving the glycosidic bond and separating the monosaccharides. This dynamic between formation and cleavage of glycosidic bonds is fundamental to carbohydrate metabolism and the biological functions of sugars.
As we delve deeper into the study of glycosidic bonds, we will explore various types and their implications in biological systems, enhancing our understanding of carbohydrate chemistry.