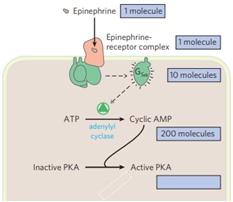
In the study of biosignaling pathways, a key focus is on G protein-coupled receptors (GPCRs) and their role in signal transduction. One significant pathway involves the stimulatory adenylate cyclase GPCR, which activates the enzyme adenylate cyclase. This enzyme catalyzes the conversion of ATP (adenosine triphosphate) into cyclic adenosine monophosphate (cAMP), a crucial secondary messenger in cellular signaling.
The structure of cAMP is closely related to ATP, differing primarily in that cAMP contains a single phosphate group in a cyclic form, while ATP has three phosphate groups. The reaction facilitated by adenylate cyclase results in the release of two inorganic phosphates, leading to the formation of cAMP. This secondary messenger plays a vital role in activating protein kinase A (PKA), an enzyme that is dependent on cAMP for its activation.
cAMP functions as an allosteric activator for PKA, meaning it binds to the inactive form of the enzyme, causing a conformational change that activates PKA. Specifically, four cAMP molecules bind to the inactive PKA, leading to the activation of its regulatory subunits. This process highlights the importance of cAMP in modulating enzymatic activity and facilitating various cellular responses.
Understanding the production and function of cAMP, along with its role in activating PKA, is essential for grasping the broader implications of GPCR signaling pathways in biological systems.