Enzymes play a crucial role in biochemical reactions by lowering the activation energy required for these processes. They achieve this through various mechanisms that enhance the likelihood of substrate interactions. One primary method is by reducing the entropy of the system, which involves bringing substrates closer together to facilitate faster reactions. Additionally, enzymes properly orient substrates, ensuring that their functional groups are aligned for optimal interaction. Another important aspect is desolvation, where enzymes remove hydration shells around substrates, allowing them to reach transition states more efficiently.
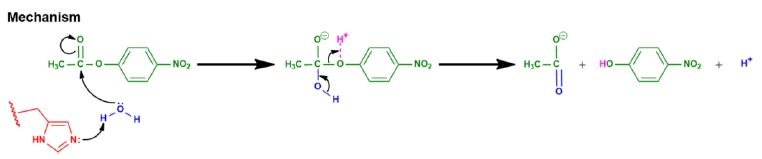
Central to all these mechanisms is the stabilization of the transition state, which is a critical point in the reaction pathway. Enzymes utilize four main types of catalysis to achieve this stabilization:
- Acid-Base Catalysis: This mechanism involves the transfer of protons (H+) to or from the substrate, which can enhance the reactivity of the substrate.
- Electrostatic Catalysis: Here, charged groups within the enzyme interact with the substrate, stabilizing charged transition states and facilitating the reaction.
- Metal Ion Catalysis: Metal ions can assist in catalysis by stabilizing negative charges, participating in redox reactions, or helping to orient substrates.
- Covalent Catalysis: In this mechanism, a transient covalent bond forms between the enzyme and the substrate, creating a reactive intermediate that accelerates the reaction.
Understanding these mechanisms is essential for grasping how enzymes function at a molecular level. The next discussion will focus specifically on acid-base catalysis, providing deeper insights into this fundamental enzymatic process.