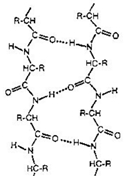
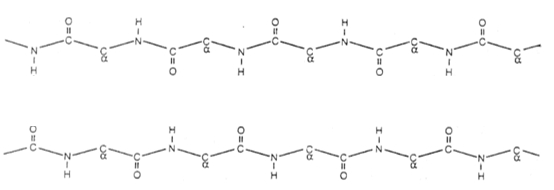
Understanding the structure of beta sheets is crucial in the study of protein secondary structures. Beta sheets can be classified into two main types: antiparallel and parallel. Antiparallel beta sheets are characterized by beta strands that run in opposite directions regarding their N- and C-terminal ends. This orientation allows for a rise per residue of 3.5 angstroms, which is consistent across all beta sheets. In an antiparallel configuration, adjacent beta strands are connected in a single polypeptide chain, forming what is known as an intra-chain antiparallel beta sheet. The arrows representing the beta strands point towards the C-terminal end, indicating the directionality of the strands.
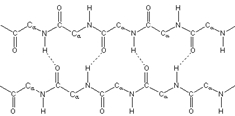
In contrast, parallel beta sheets consist of beta strands that align in the same direction concerning their N- and C-terminal ends. This arrangement results in a slightly more condensed rise per residue of 3.2 angstroms. While this is still more extended than the rise per residue of an alpha helix, which is 1.5 angstroms, it highlights the structural differences between these two types of beta sheets. Like the antiparallel configuration, parallel beta sheets also form through a continuous polypeptide chain, identified as an intra-chain parallel beta sheet.
Recognizing these structural distinctions is essential for understanding protein folding and stability. The next step in this exploration will involve examining the hydrogen bonding patterns that differentiate these two types of beta sheets, further enhancing our comprehension of protein architecture.