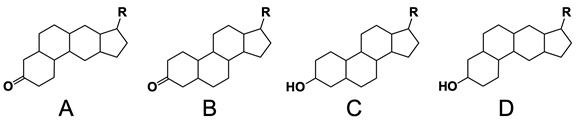
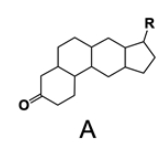
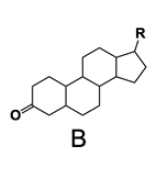
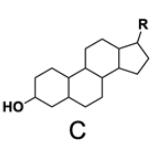
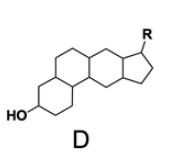
In the study of lipids, steroids represent a significant class of isoprenoid lipids characterized by their unique structure. At the core of steroids is the gonane structure, which consists of a 17-carbon tetracyclic ring. This tetracyclic ring is formed by the fusion of four rings: three six-membered rings (labeled A, B, and C) and one five-membered ring (labeled D). The term "tetracyclic" indicates the presence of four interconnected cyclic structures, which is essential for the steroid's identity.
The gonane structure is synthesized from isoprene units, which are the building blocks of isoprenoids. This biosynthetic pathway highlights the relationship between isoprenes and steroids, reinforcing the classification of steroids as isoprenoid lipids. Understanding this connection is crucial for grasping the biochemical significance of steroids in biological systems.
Among steroids, sterols are a specific subgroup that contains at least one hydroxyl group (–OH). The addition of a hydroxyl group to the gonane core transforms it into a sterol, which is vital for various biological functions. This modification exemplifies how structural variations can lead to different functional properties in lipid molecules.
As the study progresses, a particular focus will be placed on cholesterol, a well-known sterol, which plays critical roles in cellular structure and function. This foundational knowledge of steroids and their derivatives sets the stage for deeper exploration into their biological significance and applications.