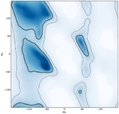
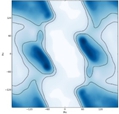
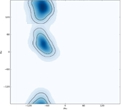
The Ramachandran plot is a valuable tool in understanding the conformational angles of amino acids in proteins. While most amino acids exhibit similar Ramachandran plots, glycine and proline stand out due to their atypical characteristics. Glycine, with the smallest R group among all amino acids, allows for greater flexibility in its phi (φ) and psi (ψ) bond rotations. This unique feature results in a Ramachandran plot where permissible angles are present in nearly every quadrant, contrasting sharply with the other 18 amino acids, which have more restricted angles due to steric hindrance.
In a typical Ramachandran plot, the white regions indicate non-permissible angles caused by steric clashes between atoms. For glycine, however, the plot reveals that it can adopt permissible bond angles across all quadrants, making it the only amino acid capable of such versatility. This flexibility enables glycine to fit into tighter spaces within protein structures, a significant advantage in various biological contexts.
Glycine's three-letter code is Gly (or GLY), and its one-letter code is G. Understanding glycine's atypical Ramachandran plot is crucial as it highlights the importance of R group size in determining the conformational possibilities of amino acids. This knowledge will be beneficial as we explore further topics in protein structure and function.