Intermolecular forces play a crucial role in determining the physical properties of substances. These forces include hydrogen bonds, dipole-dipole interactions, and van der Waals forces, each with distinct characteristics and implications.
Hydrogen bonds are a specific type of strong dipole-dipole interaction that occurs when a hydrogen atom is covalently bonded to highly electronegative atoms such as nitrogen, oxygen, or fluorine. This bond results in a significant partial positive charge on the hydrogen and a partial negative charge on the electronegative atom, leading to strong attractions between molecules. For instance, in water (H2O), each molecule can form up to four hydrogen bonds, contributing to its unique properties, such as high boiling and melting points.
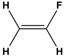
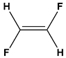
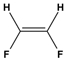
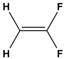
Dipole-dipole interactions arise from the presence of permanent dipoles in polar molecules. A dipole is created when there is an uneven distribution of electron density due to differences in electronegativity between two atoms. When two polar molecules approach each other, the positive end of one dipole is attracted to the negative end of another, resulting in a dipole-dipole interaction. For example, in carbonyl compounds, the oxygen atom creates a partial negative charge, while the carbon atom has a partial positive charge, leading to attractive interactions between different molecules.
Van der Waals forces, also known as London dispersion forces, are weaker interactions that occur between all molecules, regardless of polarity. These forces arise from temporary fluctuations in electron density, which can create instantaneous dipoles even in nonpolar molecules. For example, in hydrogen gas (H2), although the molecules are nonpolar and have no permanent dipoles, they can still experience van der Waals forces due to these fleeting dipoles.
Understanding these intermolecular forces is essential for predicting the behavior of substances in different states of matter and their interactions in various chemical processes.