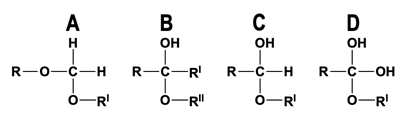
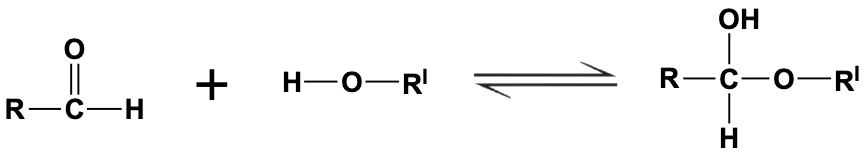
To understand hemiacetals and hemiketals, it's essential to first grasp the cyclization of monosaccharides, which occurs through a nucleophilic addition reaction. In this process, alcohol groups act as nucleophiles, while the carbonyl groups of aldehydes or ketones serve as electrophiles. This interaction leads to the formation of an anomeric carbon during the cyclization of the monosaccharide.
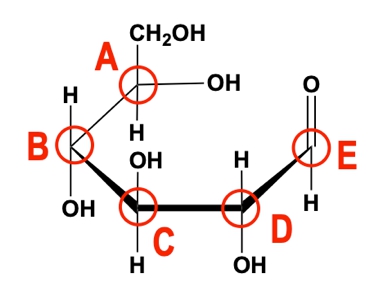
In the case of an aldehyde, the alcohol group (nucleophile) attacks the electrophilic carbonyl carbon, resulting in a cyclic structure known as a pyranose, characterized by a six-membered ring. The anomeric carbon, which is pivotal in this structure, is the carbon that was originally part of the carbonyl group and is now bonded to two oxygen atoms: one from the ring and one from the alcohol group.
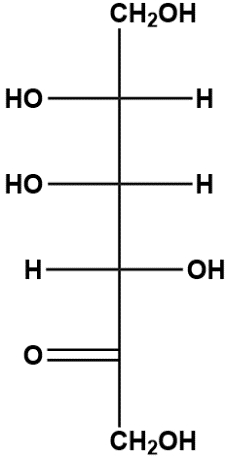
Similarly, when a ketone is involved, the reaction follows the same principle, with the alcohol group reacting with the ketone's carbonyl group. This leads to the formation of a furanose, a five-membered ring structure. Again, the anomeric carbon is formed, which retains the same characteristics as in the aldehyde reaction, being the carbon that was part of the carbonyl group and now bonded to two oxygen atoms.
Understanding these reactions is crucial as they lay the groundwork for distinguishing between hemiacetals and hemiketals, which will be explored further in subsequent discussions.