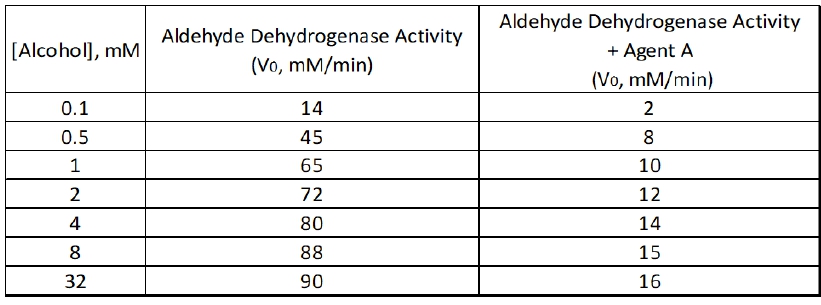
Reversible inhibition is a crucial concept in enzyme kinetics, involving four primary types of inhibitors: competitive, uncompetitive, mixed, and non-competitive. Understanding how these inhibitors affect enzyme activity is essential for grasping the dynamics of biochemical reactions.
Competitive inhibitors are unique because they compete directly with the substrate for binding to the active site of the enzyme. This competition means that increasing the substrate concentration can effectively outcompete the inhibitor, resulting in no change to the maximum reaction velocity (\(V_{max}\)). Thus, the overall effect of competitive inhibitors on \(V_{max}\) is that it remains unchanged, while the apparent Michaelis constant (\(K_m\)) increases, indicating a decreased binding affinity as represented by an upward arrow.
In contrast, uncompetitive inhibitors bind only to the enzyme-substrate complex, leading to a decrease in both \(V_{max}\) and \(K_m\). The relationship here is that as the concentration of uncompetitive inhibitor increases, the enzyme's ability to convert substrate to product diminishes, resulting in a lower \(V_{max}\) and a lower \(K_m\), which suggests an increased binding affinity.
Mixed inhibitors exhibit characteristics of both competitive and uncompetitive inhibitors. They can bind to either the free enzyme or the enzyme-substrate complex. Depending on the relative values of the inhibition constants (\(\alpha\) and \(\alpha'\)), mixed inhibitors can either increase or decrease \(K_m\). If \(\alpha\) is greater than \(\alpha'\), \(K_m\) increases, indicating a worse binding affinity. Conversely, if \(\alpha\) is less than \(\alpha'\), \(K_m\) decreases, suggesting a better binding affinity. Regardless of the effect on \(K_m\), mixed inhibitors always decrease \(V_{max}\).
Non-competitive inhibitors, a subtype of mixed inhibitors, have equal effects on both the free enzyme and the enzyme-substrate complex, leading to a situation where \(K_m\) remains unchanged while \(V_{max}\) decreases. This means that while the binding affinity is unaffected, the overall reaction rate is diminished.
In summary, the effects of reversible inhibitors on enzyme kinetics can be summarized as follows: competitive inhibitors do not change \(V_{max}\) but increase \(K_m\); uncompetitive inhibitors decrease both \(V_{max}\) and \(K_m\); mixed inhibitors can either increase or decrease \(K_m\) while always decreasing \(V_{max}\); and non-competitive inhibitors leave \(K_m\) unchanged but decrease \(V_{max}\). Understanding these relationships is vital for predicting how different inhibitors will influence enzyme activity in various biochemical contexts.