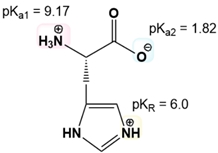
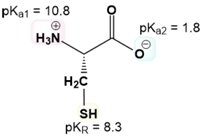
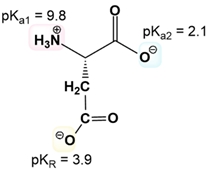
Calculating the isoelectric point (pI) of amino acids with ionizable R groups involves a more complex process than for those with non-ionizable R groups due to the presence of three pKa values instead of two. The isoelectric point is determined by averaging two of these pKa values, but the challenge lies in identifying which two to average.
To navigate this process, one can follow a systematic approach consisting of four key steps. First, it is essential to understand the net charge of both the protonated and deprotonated forms of the amino acids. A helpful mnemonic, "Yucky Crazy Dragons Eat Knights Riding Horses," can assist in recalling the seven amino acids with ionizable R groups and their respective ionization characteristics.
Next, the three pKa values should be ordered from smallest to largest. The middle pKa serves as a guide for the subsequent steps. The third step involves determining the net charge of the predominant amino acid structure at a chosen pH that lies between adjacent pKa values. This requires comparing the selected pH to the pKa values to ascertain whether the predominant form is protonated or deprotonated.
Finally, the isoelectric point is calculated by averaging the middle pKa with the pKa that corresponds to the region where the predominant structure exhibits a net charge of zero. For example, when calculating the pI for tyrosine, one would identify its three pKa values, determine the appropriate pH range, and find the net charge of the predominant structure. If the net charge is zero, the corresponding pKa values are averaged to yield the isoelectric point.
In summary, understanding the ionization behavior of amino acids, correctly ordering their pKa values, and determining the net charge at specific pH levels are crucial steps in calculating the isoelectric point for amino acids with ionizable R groups. This systematic approach not only clarifies the process but also enhances comprehension through practice and application.