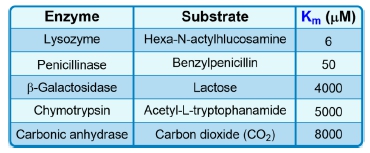
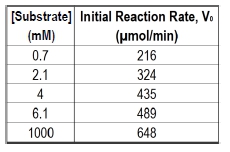
The Michaelis constant, abbreviated as km, is a crucial concept in enzyme kinetics that represents a specific substrate concentration. It is defined as the substrate concentration at which the initial reaction velocity (v0) is half of the theoretical maximum reaction velocity (vmax). This relationship indicates that at km, half of the enzyme's active sites are occupied by substrate, forming the enzyme-substrate complex.
In graphical representations of enzyme kinetics, the vmax serves as a horizontal asymptote, indicating the maximum rate of reaction that can be achieved under saturating substrate conditions. To find the km, one can determine the vmax and then calculate half of that value, which corresponds to the substrate concentration needed to occupy half of the available active sites.
It is important to note that while km is a specific substrate concentration, it is not equal to half of vmax; rather, it is the concentration at which the reaction velocity reaches that half-maximal point. The units of km are typically expressed in molarity (M), reflecting its nature as a concentration measurement.
Additionally, the km value is an intrinsic property of the enzyme, meaning it remains constant regardless of the enzyme concentration. However, it can vary under different environmental conditions, such as changes in pH, temperature, or solvent composition. Understanding the km is essential for studying enzyme behavior and kinetics, as it provides insight into the efficiency and affinity of enzymes for their substrates.