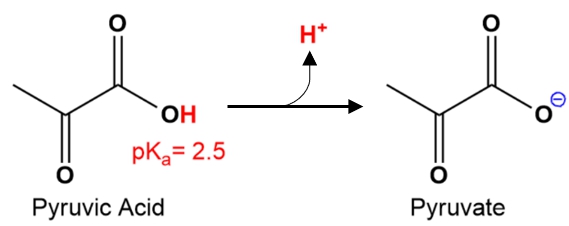
Understanding the predominant species of a molecule is essential in chemistry, particularly when dealing with acids and their conjugate bases. The Henderson-Hasselbalch equation plays a crucial role in this determination, as it expresses the relationship between the concentration of the conjugate base and the conjugate acid. This equation is given by:
\[ pH = pK_a + \log\left(\frac{[A^-]}{[HA]}\right) \]
In this equation, [A^-] represents the concentration of the conjugate base, while [HA] denotes the concentration of the conjugate acid. The predominant species refers to the most abundant form of a molecule present under specific conditions, which is influenced by the pH of the solution and the pKa of the acid.
The relationship between pH and pKa is critical; when the pH of the solution is lower than the pKa, the acidic form (HA) predominates. Conversely, when the pH is higher than the pKa, the conjugate base (A-) becomes the predominant species. This concept is vital for predicting the behavior of acids in various chemical environments.
In summary, by analyzing the pH of a solution alongside the pKa values of acids, one can effectively determine which species is predominant, enhancing the understanding of acid-base chemistry.