Multiple Choice
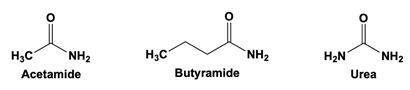
Which molecule most easily diffuses across a biological membrane's lipid bilayer, without facilitation?
 Verified step by step guidance
Verified step by step guidance
 4:18m
4:18mMaster Biological Membrane Transport with a bite sized video explanation from Jason
Start learning
