Multiple Choice
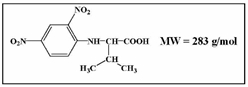
After purifying a protein, you react the protein with 1-fluoro-2,4-dinitrobenzene (FDNB or Sanger's reagent) then with 6M HCl. You obtain DNP-Arg and DNP-Asp. Which of the following is an appropriate conclusion of the results?
 Verified step by step guidance
Verified step by step guidance