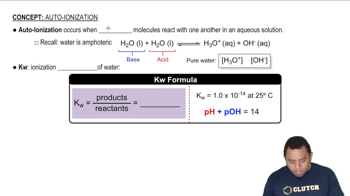
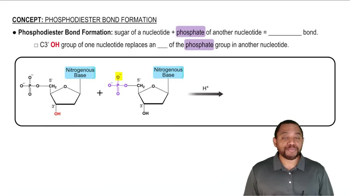
Another endoprotease is trypsin. Trypsin hydrolyzes peptide bonds on the carboxyl side of lysine and arginine. If the following peptide sequence is hydrolyzed by trypsin, how many fragments will there be? Use the three-letter amino acid abbreviations to write the fragments out.
Ala-Phe-Lys-Cys-Gly-Asp-Arg-Leu-Leu-Phe-Gly-Ala