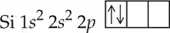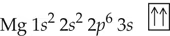Back
BackProblem 5
Locate aluminum in the periodic table and give its group number and period number.
Problem 14
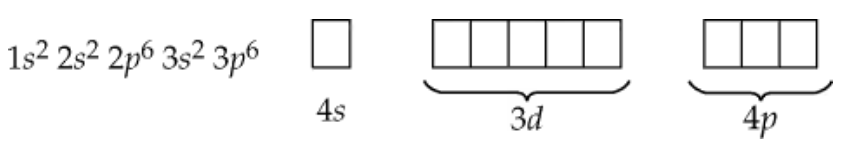
How many electrons are present in an atom in which the first and second shells and the 3s subshell are filled? Name the element.
Problem 15
An element has completely filled n = 1 and n = 2 shells and has six electrons in the n = 3 shell. Identify the element and its major group (i.e., main group, transition, etc.). Is it a metal or a nonmetal? Identify the orbital in which the last electron is found.
Problem 21
For chlorine, identify the group number, give the number of electrons in each occupied shell, and write its valence-shell configuration.
Problem 28
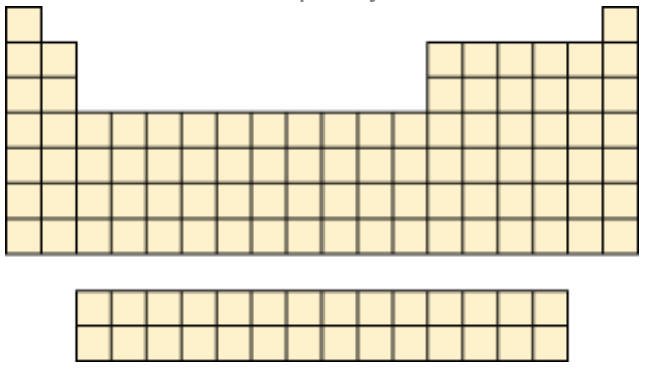
Use the following blank periodic table to show where the elements matching the following descriptions appear.
a. Elements with the valence-shell electron configuration ns2 np5
b. An element whose third shell contains two p electrons
c. Elements with a completely filled valence shell
Problem 30
Use the following orbital-filling diagram to show the electron configuration for As:
Problem 32
How do atoms of different elements differ?
Problem 34
Find the mass in atomic mass units of the following:
a. 1 O atom, with a mass of 2.66 × 10-23 g
b. 1 Br atom, with a mass of 1.31 × 10-22 g
Problem 37
How many O atoms of mass 15.99 amu are in 15.99 g of oxygen?
Problem 40
Where within an atom are the three types of subatomic particles located?
Problem 51
Why does the fourth period in the periodic table contain 18 elements?
Problem 55
Answer the following questions for the elements from cerium through lutetium:
a. Are they metals or nonmetals?
b. To what general class of elements do they belong?
c. What subshell is being filled by electrons in these elements?
Problem 65
What is the total number of orbitals in the third shell? The fourth shell?
Problem 66
How many subshells are there in the third shell? The fourth shell? The fifth shell?
Problem 70
Use arrows to show electron pairing in the valence p subshell of
a. Sulfur
b. Bromine
c. Silicon
Problem 85a
What is the mass (in amu and in grams) of a single atom of Carbon-12?
Problem 85b
What is the mass (in grams) of 6.02 × 1023 atoms of Carbon-12?
Problem 86
An unidentified element is found to have an electron configuration by shell of 2 8 18 8 2. To what group and period does this element belong? Is the element a metal or a nonmetal? How many protons does an atom of the element have? What is the name of the element? Write its electron-dot symbol.
Problem 90
What is wrong with the following electron configurations?
a. Ni 1s2 2s2 2p6 3s2 3p6 3d10
b. N 1s2 2p5
c.
d.
Problem 98
Look again at the trends illustrated in Figures 2.3 and 2.4.
a. How do the peaks/valleys correlate with locations in the periodic table?
b. Are there other chemical properties that also exhibit periodic trends? What are they?
<IMAGE>