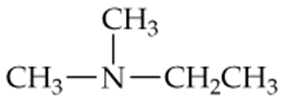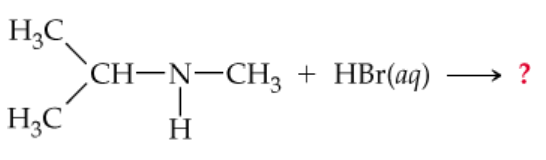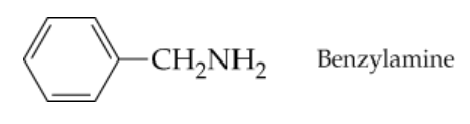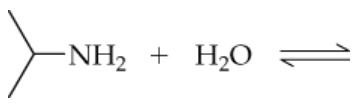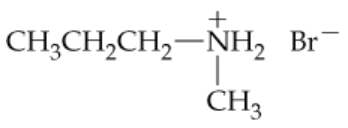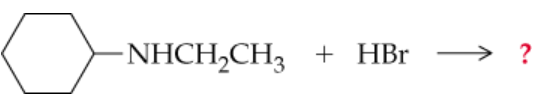Back
BackProblem 1a
Identify the following compounds as primary, secondary, or tertiary amines.
a. CH3(CH2)4CH2NH2
Problem 1b
Identify the following compounds as primary, secondary, or tertiary amines.
b. CH3CH2CH2NHCH(CH3)2
Problem 1c
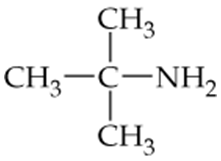
Identify the following compounds as primary, secondary, or tertiary amines.
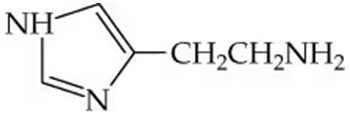
c.
Problem 2a
What are the names of these amines?
a. (CH3CH2CH2)2NH
Problem 2c
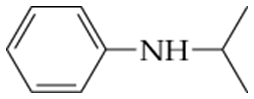
What are the names of these amines?
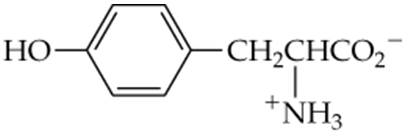
c.
Problem 3d
Draw structures corresponding to the following names:
d. 4-Amino-2-butanol
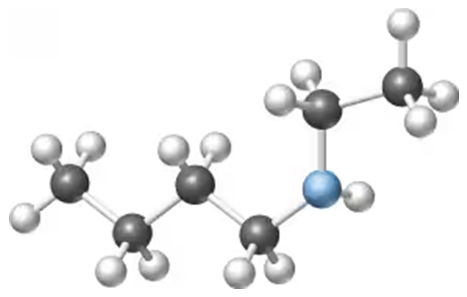
Problem 6
Draw the condensed and line formula of the molecule in the margin. Is it a primary, secondary, or tertiary amine? Why?
Problem 7a
Arrange the following compounds in order of increasing boiling point. Explain why you placed them in that order.
a.
Problem 8
Draw the structures of (a) ethylamine and (b) trimethylamine. Use dashed lines to show how they would form hydrogen bonds to water molecules.
Problem 9
Provide compounds that fit the following descriptions:
a. Two amines that are gases at room temperature
b. A heterocyclic amine
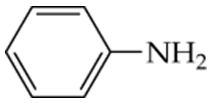
c. A compound with an amine group on an aromatic ring
Problem 11
Which of the following compounds are heterocyclic nitrogen compounds?
a.
b.
c.
d.
Problem 12a
Write an equation for the acid-base equilibrium of:
a.Pyrrolidine and water
Label each species in the equilibrium as either an acid or a base.
Problem 13a
Complete the following equations:
a.
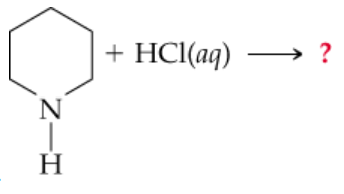
Problem 13c
Complete the following equations:
c.
Problem 15
Which is the stronger base in each pair?
a. Ammonia or ethylamine
b.Triethylamine or pyridine
Problem 17a
Write the structures of the following compounds:
a. Butyldiethylammonium bromide
Problem 21
Write the structure of benzylamine hydrochloride in two different ways, and name the hydrochloride as an ammonium salt.
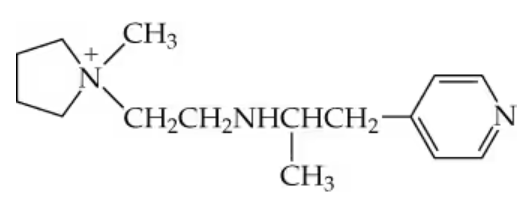
Problem 23a
a. For the compound above, identify each nitrogen as either a primary, secondary, tertiary, quaternary, or aromatic amine.
Problem 24a
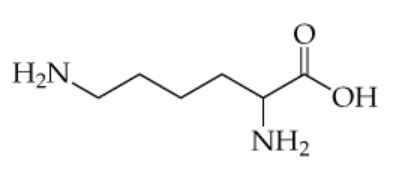
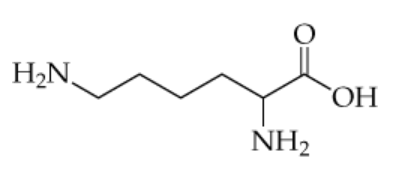
The structure of the amino acid lysine (in its uncharged form) is shown below.
a. Which amine groups would be able to participate in hydrogen bonding?
Problem 24b
The structure of the amino acid lysine (in its uncharged form) is shown below.
b. Is lysine likely to be water-soluble? Explain.
Problem 26
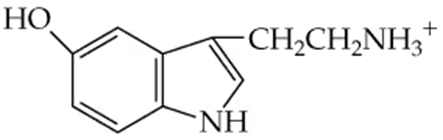
Explain what bonds must be made or broken and where the electrons go when the hydrogen-bonded water between the two amines shown on page 507 reacts to form an amine, ammonium ion, and OH⁻.
Problem 28b
Complete the following equations:
b.
Problem 30a
Draw the structures corresponding to the following names:
a. N-Methylpentylamine
Problem 32b
Name the following amines, and identify them as primary, secondary, or tertiary:
b.
Problem 35a
Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
a.
Problem 36a
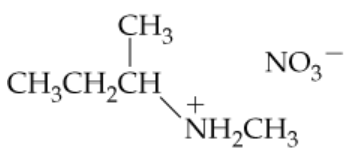
Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
a.
Problem 36c
Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
c. N-Butyl-N-isopropylhexylammonium chloride
Problem 38
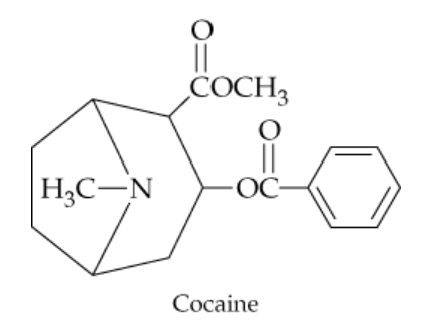
Identify the functional groups in cocaine
Problem 40
Draw the structures of the ammonium ions formed when the amines in Problem 16.30 are treated with acid.
a. N-Methylpentylamine
b. N-Ethylcyclobutylamine
c. p-Propylaniline
Problem 41a
Complete the following equations (hint: remember that a nitrogen with three groups bound to it has a lone pair and one with four does not):
a.