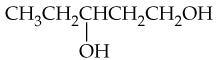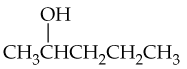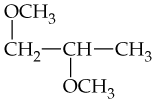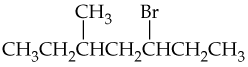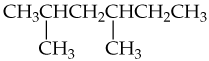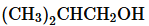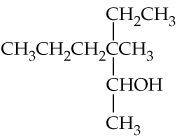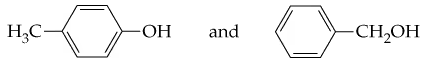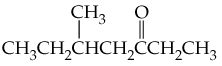Back
BackProblem 2
Ethers have some slight solubility in water. Explain this using the concept of hydrogen bonding.
Problem 6
Rank the following according to boiling point, highest to lowest:
a. CH3CH2CH2OH
b. CH3CH2(OH)CH2OH
c. CH3CH2CH3
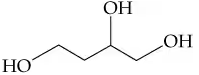
d. CH2(OH)CH(OH)CH2OH
Problem 7c
For each of the following molecules, (i) redraw using line structure format, (ii) identify its hydrophobic and hydrophilic parts, and (iii) predict its solubility in water.
c.
Problem 8b
What alkenes might be formed by dehydration of the following alcohols? If more than one product is possible in a given case, indicate which is major.
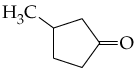
b.
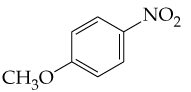
Problem 9b
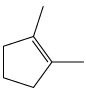
What alcohols yield the following alkenes as the major product on dehydration?
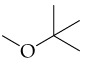
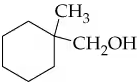
b.
Problem 11
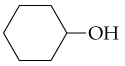
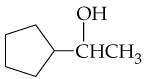
What products would you expect from oxidation of the following alcohols?
a. CH3CH2CH2OH
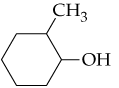
b.
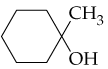
c.
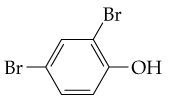
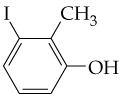
Problem 13
From what alcohols might the following carbonyl-containing products have been made (red = O, reddish-brown = Br)?
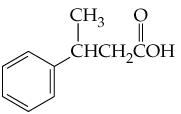
(a) <IMAGE>
(b) <IMAGE>
Problem 14
Draw structures for the following:
a. 2,4-Dinitrophenol
b. m-Ethylphenol
Problem 15
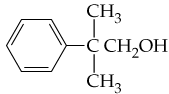
Name the following compounds:
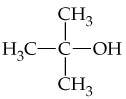
a.
b.
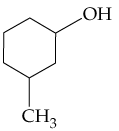
Problem 16
Name the following compounds:
a.
b.
c.
Problem 17
What disulfides would you obtain from oxidation of the following thiols?
a. CH3CH2CH2SH
b. 3-Methyl-1-butanethiol (skunk scent)
Problem 18
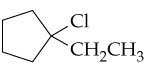
Give systematic names for the following alkyl halides:
a.
b.
Problem 19
2-Aminopropane is an achiral molecule, but 2-aminobutane is chiral. Explain.
Problem 20
Which of the following molecules are chiral? (Hint: Draw each molecule and analyze it as illustrated in Worked Example 14.2.)
a. 3-Chloropentane
b. 2-Chloropentane
c.
Problem 22
Predict the product of the following reaction:
<IMAGE>
Problem 24
The compound pictured here is a thiol. (a) Draw its line structure, and (b) draw the structure of the disulfide formed when it is treated with an oxidizing agent (yellow = S).
<IMAGE>
Problem 34a
Draw structures corresponding to the following names:
a. 2,4-Dimethyl-2-heptanol
Problem 34b
Draw structures corresponding to the following names:
b. 2,2-Diethylcyclohexanol
Problem 34f
Draw structures corresponding to the following names:
f. 3,3-Dimethyl-1,6-heptanediol
Problem 35c
Draw structures corresponding to the following names:
c. Phenyl tert-butyl ether
Problem 35e
Draw structures corresponding to the following names:
e. 2,4-Dimethoxy-3-methylpentane
Problem 35f
Draw structures corresponding to the following names:
f. 3-Methoxy-4-methyl-1-pentene
Problem 36a
Identify each alcohol named in Problem 14.32 as primary, secondary, or tertiary.
a.
b.
c.
d.
e.
f.
Problem 38
Arrange the following 6-carbon compounds in order of their expected boiling points, and explain your ranking:
a. Hexane
b. 1-Hexanol
c. Dipropyl ether (CH3CH2CH2—O—CH2CH2CH3)
Problem 40c
Draw the structures of the aldehydes that might be oxidized to yield the following carboxylic acids:
c. CH3CH=CHCOOH
Problem 43
What type of product is formed on reaction of an alcohol with Na metal?
Problem 44
Assume that you have samples of the following two compounds, both with formula C7H8O. Both compounds dissolve in ether, but only one of the two dissolves in aqueous NaOH. How could you use this information to distinguish between them?
Problem 45
Which of the following alcohols can undergo oxidation? Draw the line structure of the product expected for those that can. Assume an excess of oxidizing agent is present.
a.
b.
c.
Problem 46e
The following alkenes can be prepared by dehydration of an appropriate alcohol. Show the structure of the alcohol in each case that would provide the alkene shown as the major product.
e. 1,4-Pentadiene
Problem 49
What alcohols would you oxidize to obtain the following carbonyl compounds?
a.
b.
c.