Back
BackProblem 28
One of these pictures represents a solution of HCl and one represents a solution of H2SO4. Which is which?
a. <IMAGE>
b. <IMAGE>
Problem 34
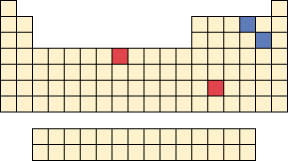
The elements in red in the periodic table can form cations having more than one charge. Write the formulas and names of the compounds that are formed between the red cations and the blue anions depicted in the periodic table.
Problem 52
Which of the following ions are likely to form? Explain.
a. Li2+
b. K-
c. Mn3+
d. Zn4+
e. Ne+
Problem 55
Write the electron configurations of Co, Co2+, and Co3+.
Problem 57a
Write equations for the loss of an electron by a K atom and the gain of an electron by a K+ ion.
Problem 74
What is the difference between an acid and a base?
Problem 76
Write equations to show how the substances listed in Problem 3.75 give ions when dissolved in water.
a. H2CO3
b. HCN
c. Mg(OH)2
d. KOH
Problem 78
Explain why the hydride ion, H-, has a noble gas configuration.