Back
BackProblem 2a
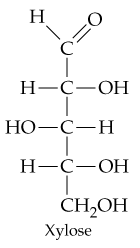
Draw the structures of an aldopentose.
Problem 2b
Draw the structures of a ketohexose.
Problem 5
Aldoheptoses have five chiral carbon atoms. What is the maximum possible number of aldoheptose stereoisomers? Draw all of the aldoheptose stereoisomers.
Problem 6a
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
a.
Problem 6b
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.
Problem 7
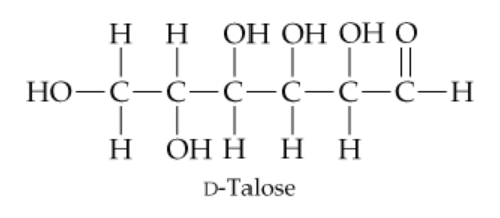
D-Talose, a constituent of certain antibiotics, has the open-chain structure shown next. Draw d-talose in its cyclic hemiacetal form.
Problem 8
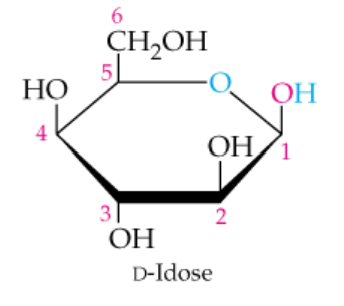
The cyclic structure of D-idose, an aldohexose, is shown in the margin. Convert this to the straight-chain Fischer projection structure.
Problem 9a
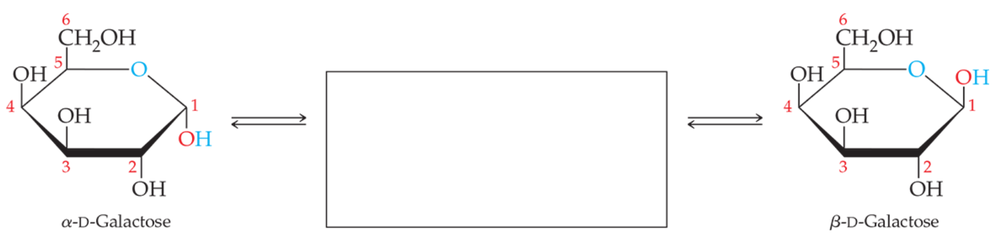
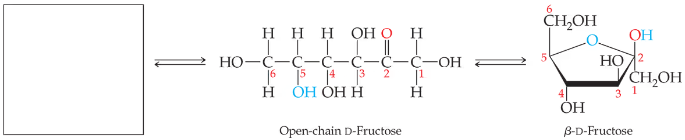
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
a.
Problem 9b
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
b.
Problem 11
In the monosaccharide hemiacetal shown below number all the carbon atoms, identify the anomeric carbon atom, and identify it as the α or β anomer.
Problem 13d
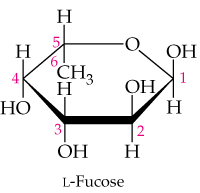
L-Fucose is one of the naturally occurring L monosaccharides. It is present in the short chains of monosaccharides by which blood groups are classified. Compare the structure of L-fucose shown in the margin with the structures of α- and β-D-galactose and answer the following questions.
d. "Fucose” is a common name. Is 6-deoxy-L-galactose a correct name for fucose? Why or why not?
Problem 14
Draw the structure of the α and β anomers that result from the reaction of methanol and ribose. Are these compounds acetals or hemiacetals?
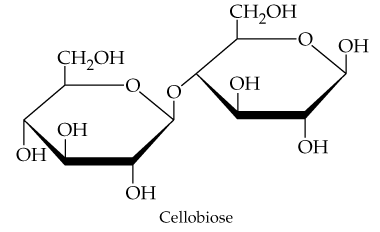
Problem 15
How would you classify the link between the monosaccharides in cellobiose?
Problem 20
During the digestion of starch from potatoes, the enzyme α-amylase catalyzes the hydrolysis of starch into maltose. Subsequently, the enzyme maltase catalyzes the hydrolysis of maltose into two glucose units. Write an equation (in words) for the enzymatic conversion of starch to glucose. Classify each of the carbohydrates in the equation as a disaccharide, monosaccharide, or polysaccharide.
Problem 21
Identify the following as diastereomers, enantiomers, and/or anomers.
(a) β-D-fructose and β-D-fructose
(b) D-galactose and L-galactose
(c) L-allose and D-glucose (both aldohexoses)
Problem 22a
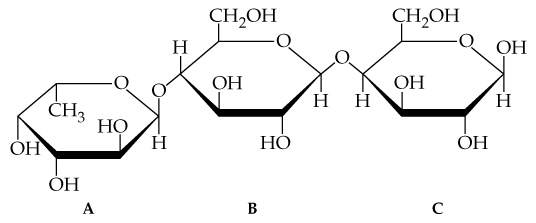
Consider the trisaccharide A, B, C shown in Problem 20.23.
a. Identify the hemiacetal and acetal linkages.
Problem 22c
Consider the trisaccharide A, B, C shown in Problem 20.23.
c. State the numbers of the carbon atoms that form glycosidic linkages between monosaccharide A and monosaccharide B.
Problem 23c
Hydrolysis of both glycosidic bonds in the following trisaccharide A, B, C yields three monosaccharides.
c. Draw the Fischer projections for the three monosaccharides.
Problem 25
Are one or more of the disaccharides maltose, lactose, cellobiose, and sucrose part of the trisaccharide in Problem 20.23? If so, identify which disaccharide and its location. (Hint: Look for an α-1,4 link, β-1,4 link, or 1,2 link, and then determine if the correct monosaccharides are present.)
Problem 27
In solution, glucose exists predominantly in the cyclic hemiacetal form, which does not contain an aldehyde group. How is it possible for mild oxidizing agents to oxidize glucose?
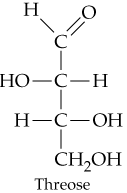
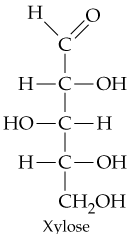
Problem 31c
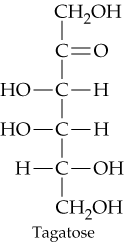
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
c.
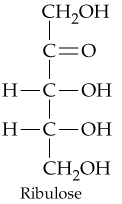
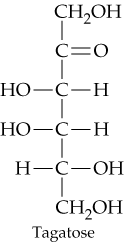
Problem 31d
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
d.
Problem 32
How many chiral carbon atoms are present in each of the molecules shown in Problem 20.31?
a.
b.
c.
d.
Problem 35
Draw the open-chain structure of a 4-carbon deoxy sugar.
Problem 36
Name four important monosaccharides and tell where each occurs in nature.
Problem 40
Only three stereoisomers are possible for 2,3-dibromo-2, 3-dichlorobutane. Draw them, indicating which pair are enantiomers (optical isomers). Why does the other isomer not have an enantiomer?
Problem 41
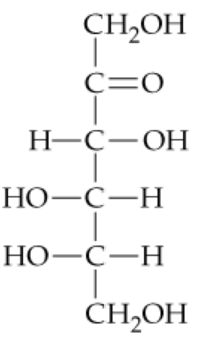
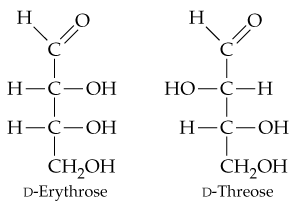
In Section 15.6, you saw that aldehydes react with reducing agents to yield primary alcohols (RCH=O → RCH2OH). The structures of two D-aldotetroses are shown. One of them can be reduced to yield a chiral product, but the other yields an achiral product. Explain.
Problem 42b
Sucrose and D-glucose rotate plane-polarized light to the right; D-fructose rotates light to the left. When sucrose is hydrolyzed, the glucose–fructose mixture rotates light to the left.
b. Why do you think the mixture is called “invert sugar”?
Problem 50
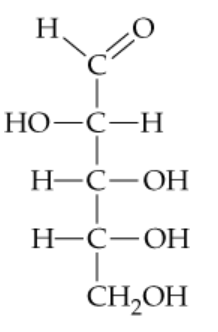
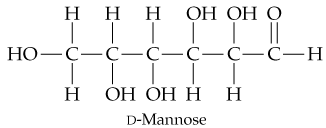
In its open-chain form, D-mannose, an aldohexose found in orange peels, has the structure shown here. Coil mannose around and draw it in the cyclic hemiacetal ⍺ and β forms.
Problem 52
Treatment of D-glucose with a reducing agent yields sorbitol, a substance used as a sugar substitute by people with diabetes. Draw the structure of sorbitol.