Back
BackProblem 8
Identify the oxidized reactant, the reduced reactant, the oxidizing agent, and the reducing agent in the following reactions:
a. Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
b. Mg(s) + Cl2(g) → MgCl2(s)
c. 2 Al(s) + Cr2O3(s) → 2 Cr(s) + Al2O3(s)
Problem 9
Potassium, a silvery metal, reacts with bromine, a corrosive, reddish liquid, to yield potassium bromide, a white solid. Write the balanced equation, and identify the oxidizing and reducing agents.
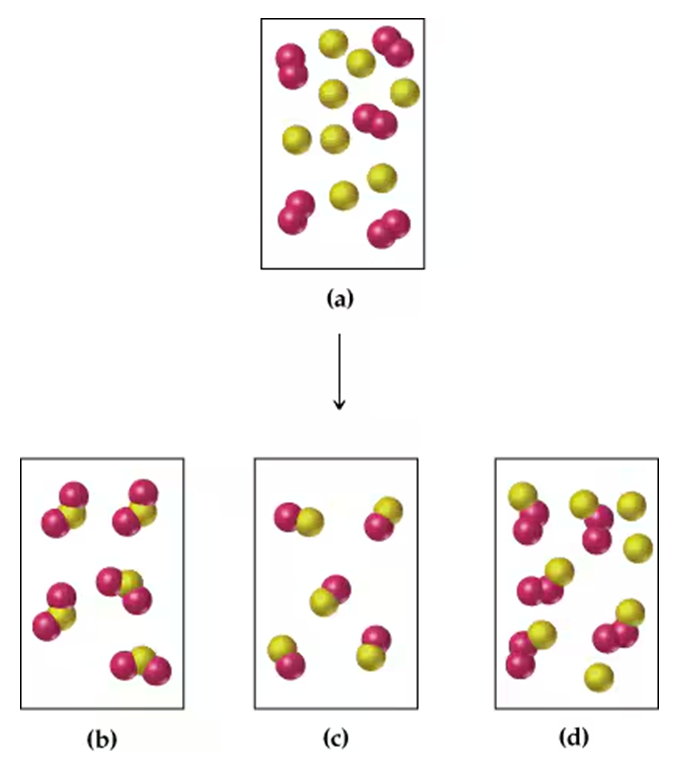
Problem 16
Assume that the mixture of substances in drawing (a) undergoes a reaction. Which of the drawings (b)–(d) represent a product mixture consistent with the law of conservation of mass?
Problem 17
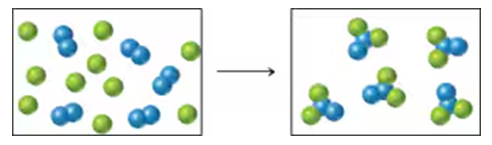
Reaction of A (green spheres) with B (blue spheres) is shown in the following diagram:
Which equation best describes the reaction?
a. A2 + 2 B → A2B2
b. 10 A + 5 B2 → 5 A2B2
c. 2 A + B2 → A2B2
d. 5 A + 5 B2 → 5 A2B2
Problem 20
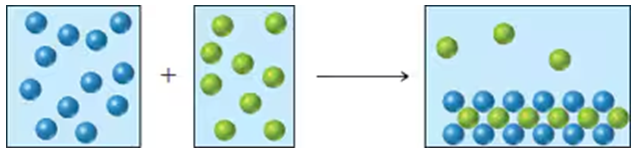
An aqueous solution of a cation (represented as blue spheres in the diagram) is allowed to mix with a solution of an anion (represented as green spheres) and the following result is obtained:
Which combinations of cation and anion, chosen from the following lists, are compatible with the observed results? Explain.
Cations: Na+, Ca2+, Ag+, Ni2+
Anions: Cl−, CO23–, CrO42–, NO3–
Problem 22
What is meant by the term 'balanced equation'?
Problem 23
Why is it not possible to balance an equation by changing the subscript on a substance, say from H2O to H2O2?
Problem 27
Which of the following equations are balanced? Balance those that need it.
a. CaC2 + 2 H2O → Ca(OH)2 +C2H2
b. C2H8N2 + 2 N2O4 → 2 N2 + 2 CO2 + 4 H2O
c. 3 MgO + 2 Fe → Fe2O3 + 3 Mg
d. N2O → N2 + O2
Problem 44a
When sodium metal is placed in water, the following change occurs: Sodium, Na(s) + Water, H2O(l) → Hydrogen, H2(g) + Sodium hydroxide, NaOH(aq)
a. Identify the reactants and products and their physical states
Problem 49
In each of the following, tell whether the substance gains electrons or loses electrons in a redox reaction:
a. An oxidizing agent
b. A reducing agent
c. A substance undergoing oxidation
d. A substance undergoing reduction
Problem 73a
High temperature combustion processes, such as in combustion engines and coal-fired power plants, can result in the reaction of nitrogen and sulfur with oxygen to form nitrogen oxides (NO𝓍) and sulfur oxides (SO𝓍), where x can vary. These NO𝓍 and SO𝓍 compounds subsequently undergo further reaction in the atmosphere to create acidic compounds that contribute to acid rain.
a. Do some research to determine the common products that are formed (i.e., what are the values of x) for the reactions of N and S with oxygen. Write balanced equations for these reactions.
Problem 75
Many pharmaceuticals are marketed with the designation "HCl" appended to the name of the drug. What does the "HCl" mean? What type of reaction would be involved in converting a drug to the HCl form? What are the advantages of this form of the drug?