If the same peptide found in Problem 18.32 is subjected to acid hydrolysis, how many fragments will result? Why?
Ala-Phe-Lys-Cys-Gly-Asp-Arg-Leu-Leu-Phe-Gly-Ala

 Verified step by step guidance
Verified step by step guidance

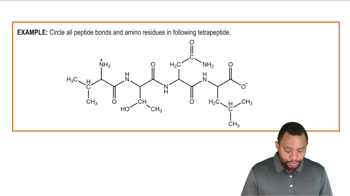
If the same peptide found in Problem 18.32 is subjected to acid hydrolysis, how many fragments will result? Why?
Ala-Phe-Lys-Cys-Gly-Asp-Arg-Leu-Leu-Phe-Gly-Ala
Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
a. Val
Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
d. Glu-Asp
Interactions of amino acids on the interior of proteins are key to the shapes of proteins. In group (a), which pairs of amino acids form hydrophobic interactions? In group (b), which pairs form ionic interactions? Which pairs in group (c) form hydrogen bonds?
a. 1 Pro . . . Phe
2 Lys . . . Ser
3 Thr . . . Leu
4 Ala . . . Gly
Draw the hexapeptide Asp-Gly-Phe-Leu-Glu-Ala in linear form showing all of the atoms, and show (using dotted lines) the hydrogen bonding that stabilizes this structure if it is part of an α-helix.
Compare and contrast the characteristics of fibrous and globular proteins. Consider biological function, water solubility, amino acid composition, secondary structure, and tertiary structure. Give examples of three fibrous and three globular proteins. (Hint: Make a table.)