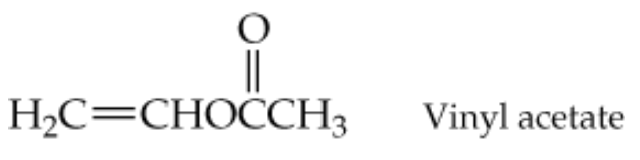Back
BackProblem 2
Draw both condensed and line structures corresponding to the following IUPAC names:
a. 3-Methyl-1-heptene
b. 4,4-Dimethyl-2-pentyne
c. 2-Methyl-3-hexene
d. 1,3,3-Trimethylcyclohexene
Problem 4
Which of the following substances exist as can cis–trans isomers? Draw both isomers for those that do.
a. 2,3-Dimethyl-2-pentene (condensed structures only)
b. 2-Methyl-2-hexene (both condensed and line structures)
c. 2-Hexene (line structures only)
Problem 7
Classify the following reactions as an addition, elimination, or substitution:
a. CH3Br + NaOH → CH3OH + NaBr
b. H2C═CH2 + HCl → CH3CH2Cl
c. CH3CH2Br → H2C═CH2 + HBr
Problem 8a
Many biological transformations can be simply classified as additions, eliminations, or substitutions. How would you classify the following reactions?
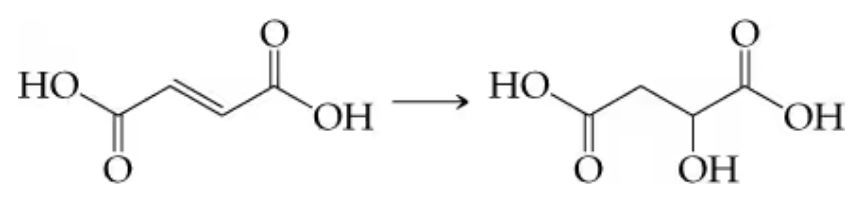
a. Fumaric acid to malic acid
Problem 8b
Many biological transformations can be simply classified as additions, eliminations, or substitutions. How would you classify the following reactions?
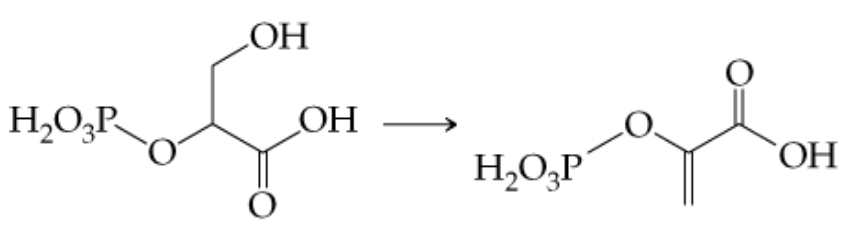
b. 2-Phosphoglyceric acid to phosphoenolpyruvic acid
Problem 11
Draw all possible products formed when 2-methyl-2-butene undergoes addition with HCl. Label them as being either the major or the minor product.
Problem 13a
In the following addition reactions, are the given alkyl halides obtained as the major products? Give a reason for your answer.
a. 3-Chloro-3-ethylpentane from addition of HCl to 3-ethyl-2-pentene
Problem 16
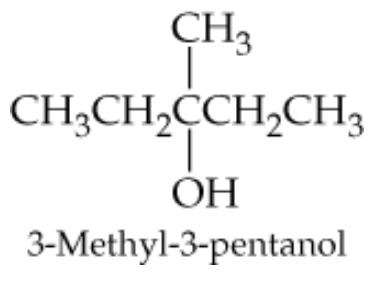
Draw the structures of the two different alkenes from which 3-methyl-3-pentanol, shown in the margin, can be made. Draw them in both condensed and line format.
Problem 17
The structure of vinyl acetate is shown below (the partial structure H2C=CH-is known as a vinyl group). When polymerized it produces poly(vinyl acetate), a polymer used for the springy soles in running shoes. Draw the structure of the polymer obtained if three vinyl acetate units underwent polymerization.
Problem 20
Draw structures corresponding to the following names (refer to Table 13.2 if necessary):
a. m-Chloronitrobenzene
b. o-Nitrotoluene
c. p-Methylaniline
d. p-Nitrophenol
Problem 23
Reaction of Br2 and FeBr3 with phenol can lead to three possible substitution products. Show the structure of each and name them.
Problem 27
Draw the product from reaction of the following substances with (1) Br2 and FeBr3 and (2) SO3 and H2SO4 catalyst (red=O):
(a) <IMAGE>
(b) <IMAGE>
Problem 28
Alkynes undergo hydrogenation to give alkanes, just as alkenes do. Draw and name the products that would result from hydrogenation of the alkynes shown in Problem 13.25.
<IMAGE>
Problem 30a
What do the terms saturated and unsaturated mean?
Problem 30b
Draw an example of a saturated four carbon compound and an unsaturated four carbon compound.
Problem 31a
What does the term 'aromatic' refer to when discussing organic molecules?
Problem 31b
What is resonance and why is it important in aromatic compounds?
Problem 33
What prefixes are used in naming the following?
a. A 1,3-disubstituted benzene
b. A 1,4-disubstituted benzene
Problem 34a
Write structural formulas for compounds that meet the following descriptions:
a. A 6-carbon alkene whose longest chain is 4 carbons in length (three possibilities)
Problem 34b
Write structural formulas for compounds that meet the following descriptions:
b. An alkyne with 5 carbons total (three possibilities)
Problem 34d
Write structural formulas for compounds that meet the following descriptions:
d. A disubstituted benzene with a total of 8 carbons (three possibilities)
Problem 35a
Write structural formulas for compounds that meet the following descriptions:
a. An alkene, C6H12, that cannot have cis–trans isomers and whose longest chain is 5 carbons long
Problem 35b
Write structural formulas for compounds that meet the following descriptions:
b. An alkene with a chemical formula of C10H12, that has cis–trans isomers and contains a benzene ring.
Problem 38a,b,c,d
Draw structures corresponding to the following IUPAC names:
a. trans-2-Pentene
b. trans-3,4-Dimethyl-3-hexene
c. 2-Methyl-1,3-butadiene
d. trans-3-Heptene
Problem 40
Seven alkynes have the formula C6H10. Draw them using line structures.
Problem 41
Draw and name all phenols with the formula C7H8O .
Problem 42
When ethylbenzene is reacted with nitric acid, three possible benzenes containing both a nitro group and an ethyl group are obtained. Draw and name them.
Problem 52
What is the difference between a substitution reaction and an addition reaction?
Problem 54
If 2-methyl-2-pentene were converted into 1-hexene, what kind of reaction would that be?
Problem 55
If bromocyclohexane were converted into cyclohexene, what kind of reaction would that be?